Your Location:Home > Products > Medical intermediate > 1,2,3,4-Tetra-O-acetyl-β-D-glucuronic acid methyl ester
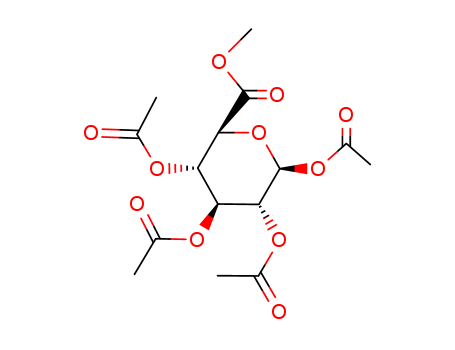

CasNo: 7355-18-2
MF: C15H20 O11
Appearance: White Powder
Synonyms: Methyl 1,2,3,4-tetra-acetyl-D-glucopyranuronate;Methyl 1,2,3,4-Tetra-O-acetyl β-D-glucuronate;
Molecular Formula: C15H20O11
Molecular Weight: 376.31
CAS Number:7355-18-2
Molecular Structure:
.png)
Specification:
|
Item |
Specification |
|
Appearance |
White to off-white powder |
|
Assay (GC),% |
≥98.0 |
|
Specific rotation [α]20D (C=1,CHCl3) |
+6.0~+11.0° |
|
Loss on drying,% |
≤0.5 |
|
Residue on ignition,% |
≤0.5 |
Glycoside pharmaceutical intermediates.
A macrocyclic conjugate of the natural d...
Novel phosphorylated glycolipids based o...
A facile and regiocontrolled procedure f...
Glucuronic acid is a key component of th...
A series of new carbohydrate-based sulph...
Synthetic immune-stimulatory drugs such ...
Carbohydrates are involved in many impor...
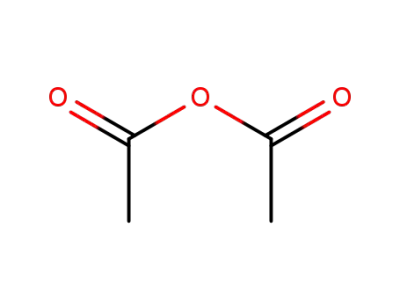
acetic anhydride

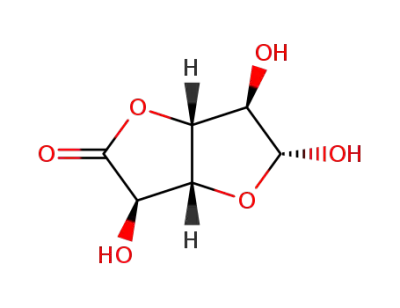
(+)-D-glucuronic acid γ-lactone

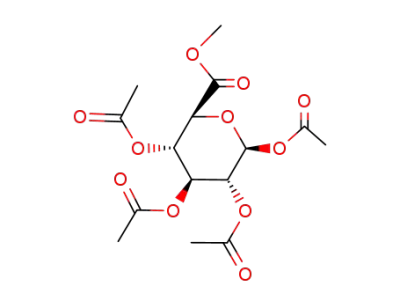
(2S,3S,4S,5R,6S)-3,4,5,6-Tetraacetoxy-tetrahydro-pyran-2-carboxylic acid methyl ester
| Conditions | Yield |
|---|---|
|
(+)-D-glucuronic acid γ-lactone; With sodium hydroxide; In methanol; at 0 - 28 ℃; for 2h;
acetic anhydride; With triethylamine; for 0.416667h; Time; Reagent/catalyst; Temperature;
|
84.31% |
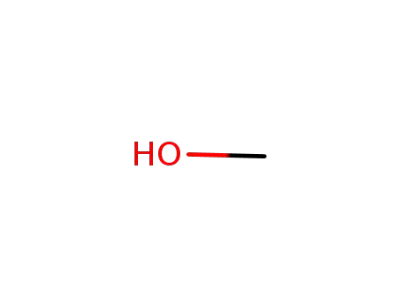
methanol

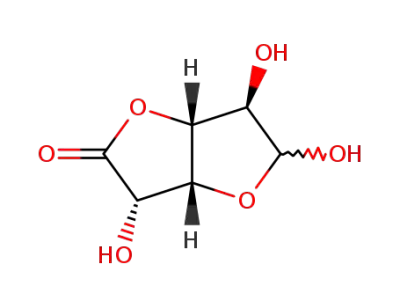
D-glucurono-6,3-lactone


acetic anhydride


(2S,3S,4S,5R,6S)-3,4,5,6-Tetraacetoxy-tetrahydro-pyran-2-carboxylic acid methyl ester
| Conditions | Yield |
|---|---|
|
methanol; D-glucurono-6,3-lactone; With sodium hydroxide; at 20 ℃; for 2h;
acetic anhydride; With pyridine; at 0 - 20 ℃; for 3h;
|
65% |
|
methanol; D-glucurono-6,3-lactone; With sodium hydroxide; at 20 ℃; for 1h;
acetic anhydride; With pyridine; at 4 ℃; for 14h;
|
57% |
|
methanol; D-glucurono-6,3-lactone; With N,N-dimethyl-ethanamine; for 16h;
acetic anhydride; With sodium acetate; for 96h;
|
39% |
|
methanol; D-glucurono-6,3-lactone; With N,N-dimethyl-ethanamine; for 3h;
acetic anhydride; With sodium acetate; for 192h;
|
35% |
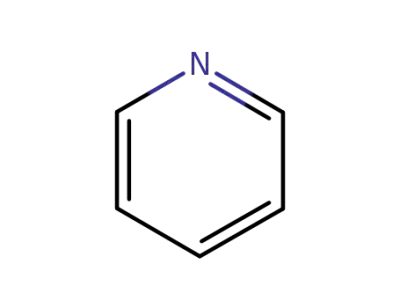
pyridine
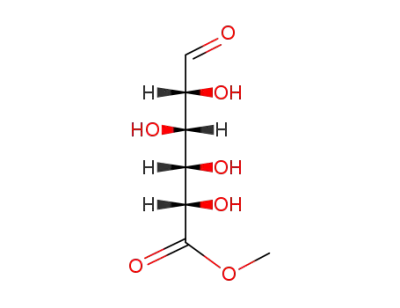
glucuronic acid methyl ester

acetic anhydride
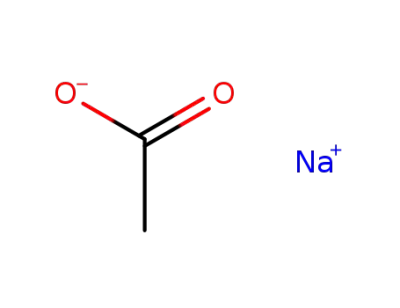
sodium acetate
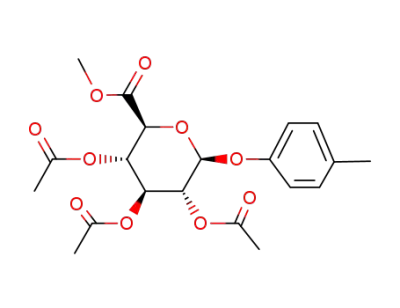
4-(methyl)phenyl-beta-D-glucopyranosiduronic acid methyl ester tetraacetate
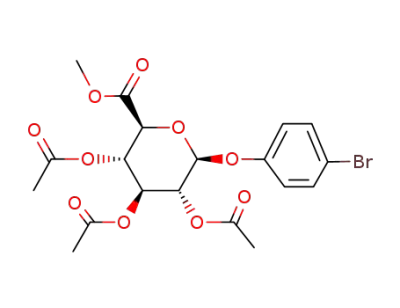
O2,O3,O4-triacetyl-O1-(4-bromo-phenyl)-β-D-glucopyranuronic acid methyl ester
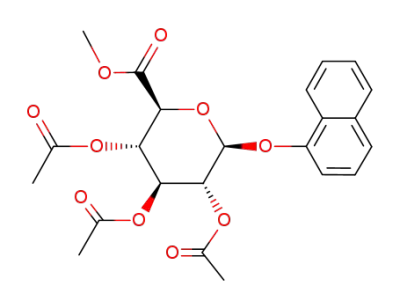
O2,O3,O4-triacetyl-O1-[1]naphthyl-β-D-glucopyranuronic acid methyl ester
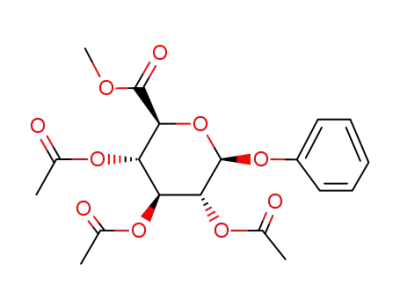
2,3,4-tri-O-acetyl-1-O-phenyl-β-D-glucopyranuronic acid methyl ester