Your Location:Home > Products > Pharmaceutical Excipient > Sucrose Octaacetate(Usp/Nf/Chp)

CasNo: 126-14-7
MF: C28H38O19
Appearance: white to creamy white powder
Packing: 25kgs/drum or as required by the customer.
Synonym: Octaacetyl Sucrose, Saccharose Octaacetate
Chemical Formula: C28H38O19
Molecular Weight: 678.6
CAS Number: 126-14-7
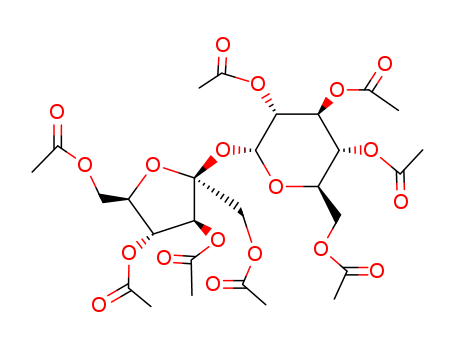
Molecular Structure:
Specification: ChP2025
|
Item |
Specification |
|
Appearance |
White powder |
|
Related substance |
|
|
Identification |
Complies |
|
Melting temperature,℃ |
≥78 |
|
Acidity,% |
≤2drops |
|
Water,% |
≤1.0 |
|
Residue on ignition,% |
≤0.5 |
|
Assay (HPLC), % |
≥98.0 |
Properties: It is white powder with a strong bitter taste. It is easily soluble in methanol, soluble in ethanol , and very slightly soluble in water. The density is 1.28; boiling point is 250 °C.
This product is used as alcohol denaturant. It also can be used for the treatment of gonarthromeningitis traditional Chinese medicine preparation placebo, coronary heart disease prescription placebo and the treatment of cardiovascular disease drugs.
It is also a good remedy for thumb sucking and nail biting in children. This product can also be used for the synthesis of sucrose polyester.
Storage: Well closed and store in a dry place.
Package: 25kgs/drum or as required by the customer.
Expiration Date: 2 years
A new phenylpropanoid glycoside, named s...
-
-
N-(3′,4′-Dihydroxy-trans-cinnamoyl)-3-(3...
-
Archetypal O-acetylation reactions of al...
(Chemical Equation Presented) Molecular ...
The solvent free per-O-acetylation of va...
A practical and highly efficient approac...
Sulfuric acid immobilized on silica gel ...
Despite the difficulty of direct β-furan...
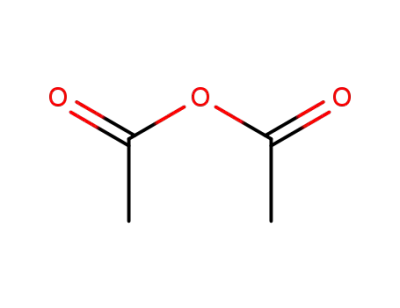
acetic anhydride

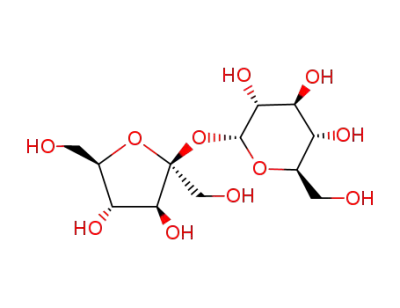
Sucrose

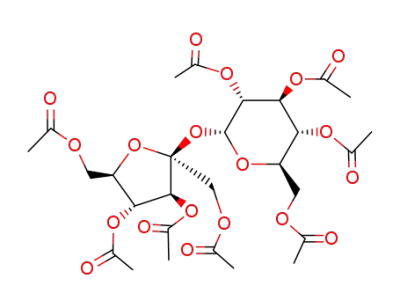
sucrose octaacetate
| Conditions | Yield |
|---|---|
|
With copper(II) perchlorate hexahydrate; In neat (no solvent); at 20 ℃; for 0.5h; Inert atmosphere;
|
97% |
|
With iodine; at 50 ℃; for 0.166667h; Microwave irradiation;
|
96% |
|
With butylmethylimidazolium dicyanamide; at 25 ℃; for 24h;
|
93% |
|
perchloric acid; at 0 - 20 ℃; for 0.166667h;
|
92% |
|
With sodium acetate; for 0.00972222h; microwave irradiation;
|
74% |
|
iron(III) chloride; at 20 ℃; for 1h;
|
65% |
|
With sodium acetate;
|
|
|
With pyridine;
|
|
|
With pyridine; N,N-dimethyl-formamide;
|
|
|
With pyridine; Yield given;
|
|
|
With pyridine; for 8h; Heating;
|
15 mg |
|
With pyridine; at 20 ℃;
|
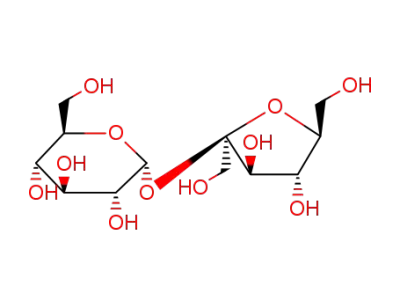
sucrose


acetic anhydride


sucrose octaacetate
| Conditions | Yield |
|---|---|
|
With lithium perchlorate; for 5h; Heating;
|
98% |
|
With 1H-imidazole; In acetonitrile; at 20 ℃; for 10h;
|
98% |
|
With Sulfuric acid immobilized on silica gel; at 20 ℃; for 1h; neat (no solvent);
|
91% |

acetic anhydride

Sucrose
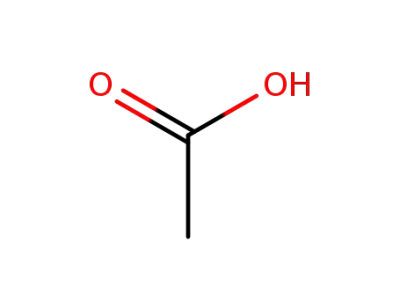
acetic acid
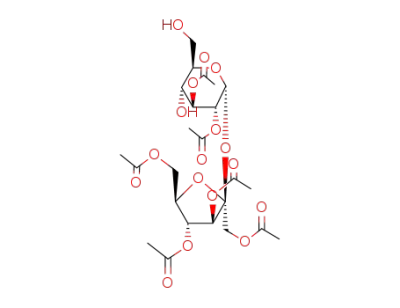
1',2,3,3',4',6'-hexa-O-acetyl sucrose
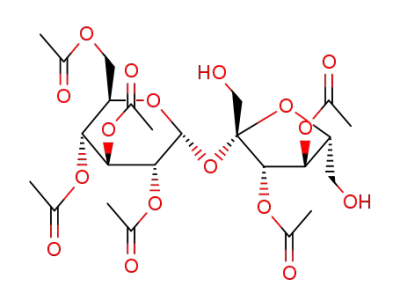
3,4,-di-O-acetyl-β-D-fructofuranosyl 2,3,4,6-tetra-O-acetyl-α-D-glucopyranoside
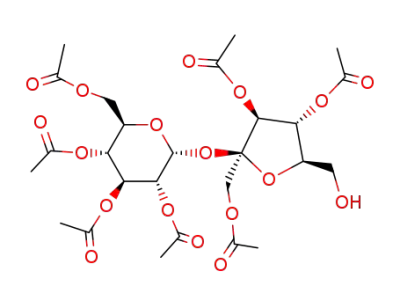
1,3,4'-tri-O-acetyl-β-D-fructofuranosyl 2,3,4,6-tetra-O-acetyl-α-D-glucopyranoside
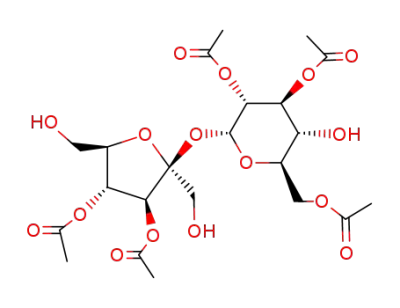
2,3,6,3',4'-pentaacetyl sucrose (6-PAS)
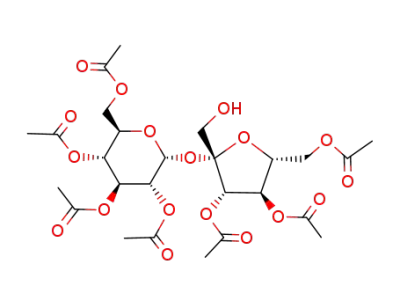
3,4,6-tri-O-acetyl-β-D-fructofuranosyl 2,3,4,6-tetra-O-acetyl-α-D-glucopyranoside