Your Location:Home > Products > Medical intermediate > Benzyl-β-L-Arabinoside
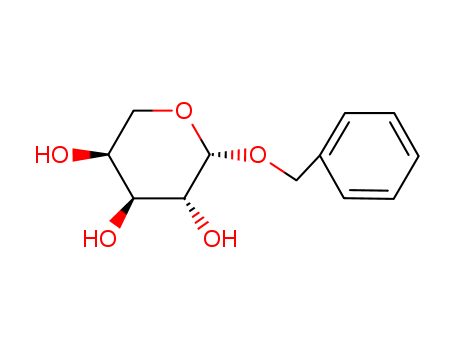

CasNo: 7473-38-3
MF: C12H16O5
Packing: 50kg/drum or as customer require.
Synonyms: Benzyl-β-L-Arabinopyranoside; Nsc400277.
Molecular Formula: C12H16O5
Molecular weight: 240.25
CAS No.: 7473-38-3
Storage: Maintain in cool, dry containment.
Package: 50kg/drum or as customer require.
Expiration Date: 2 years
Molecular structure:
Quality Standard:
|
Item |
Specification |
|
Appearance |
White crystalline powder |
|
Assay(GC),% |
≥98 |
|
Specific rotation, |
+200~+215 |
|
Melting point, ℃ |
170~178 |
|
Loss on drying, % |
≤1.0 |
|
Residue on ignition, % |
≤0.1 |
The product is a material and intermediates of pharmaceuticals. It mainly used in synthesis L-ribose, L-glycerol monophosphate, anthracycline-based glycoside and 2'-deoxy-2'-fluoro-5-methyl-β-L-arabinofuranosyluridine, etc., while it also use in synthesis disaccharide after been benzoylation.
Provided are biaryl amides and coumarin-...
The synthesis and stereochemical assignm...
The Zn-proteinase, isolated from Sacchar...
The effect of diol blocking groups, cycl...
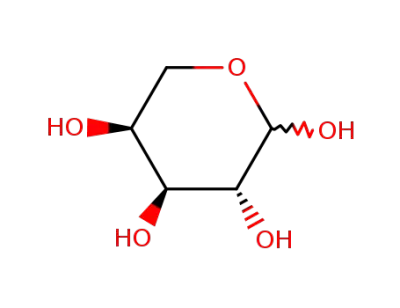
L-(+)-arabinose

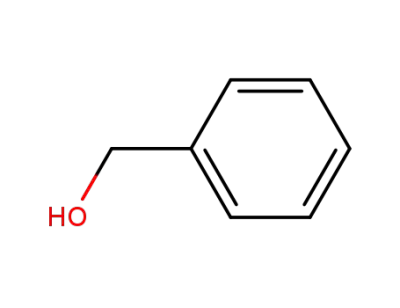
benzyl alcohol

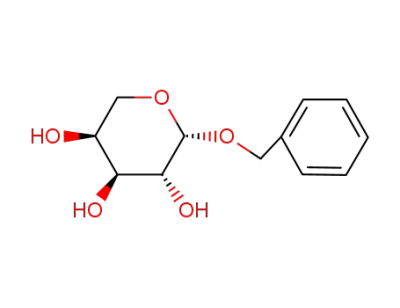
benzyl β-L-arabinopyranoside
| Conditions | Yield |
|---|---|
|
benzyl alcohol; With acetyl chloride; at 10 - 30 ℃; for 1.5h; Inert atmosphere;
L-(+)-arabinose; at 30 ℃; for 18h; Inert atmosphere;
|
91% |
|
With acetyl chloride; at 20 ℃; for 120h;
|
88% |
|
With acetyl chloride; at 20 ℃; for 120h;
|
87% |
|
With acetyl chloride; Reflux;
|
87% |
|
With hydrogenchloride; at 0 ℃; for 5h;
|
87% |
|
With acetyl chloride; 1.) 0 deg C, 2.) 50 deg C, 24 h;
|
82% |
|
With hydrogenchloride; at 100 ℃; for 3h;
|
80.8% |
|
With hydrogenchloride; for 18h; Ambient temperature;
|
75% |
|
With hydrogenchloride; In diethyl ether;
|
64% |
|
|
64% |
|
With hydrogenchloride; for 12h; 0 deg C to r.t.;
|
|
|
With hydrogenchloride; Ambient temperature;
|
12.97 g |
|
With hydrogenchloride; at 20 ℃; for 10h;
|
|
|
|
|
|
With hydrogenchloride; at 20 ℃; for 16h;
|
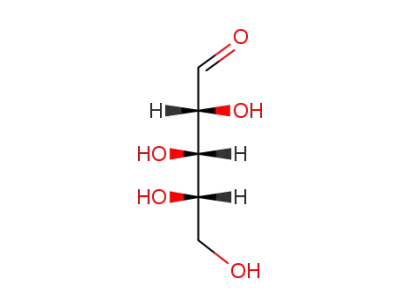
L-arabinose


benzyl alcohol


benzyl β-L-arabinopyranoside
| Conditions | Yield |
|---|---|
|
With acetyl chloride; at 20 ℃; for 120h;
|
87% |
|
With boron trifluoride diethyl etherate; at 90 - 100 ℃; for 2.5h;
|
46% |
|
With toluene-4-sulfonic acid; at 60 ℃;
|
|
|
With hydrogenchloride; at 0 - 20 ℃;
|

L-(+)-arabinose

benzyl alcohol
L-arabinose
β-L-arabinopyranose
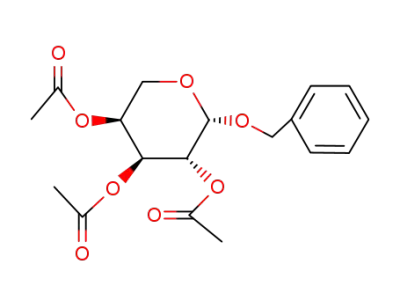
Benzyl-2,3,4-tri-O-acetyl-β-L-arabinopyranosid
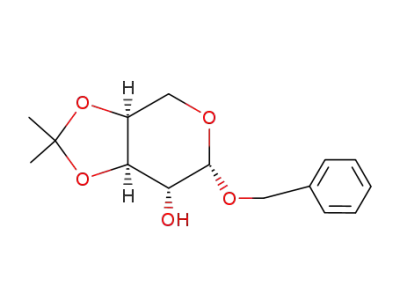
(3aS,6S,7R,7aR)-6-Benzyloxy-2,2-dimethyl-tetrahydro-[1,3]dioxolo[4,5-c]pyran-7-ol
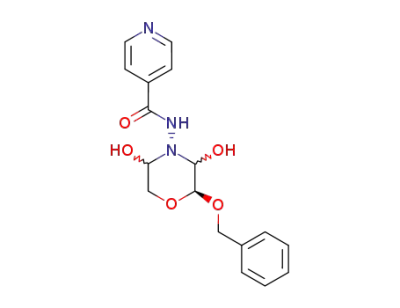
N-((2S)-2r-benzyloxy-3ξ,5ξ-dihydroxy-morpholin-4-yl)-isonicotinamide
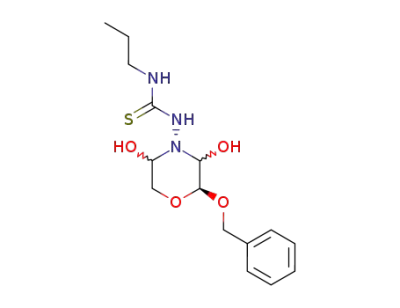
1-((2S)-2r-benzyloxy-3ξ,5ξ-dihydroxy-morpholin-4-yl)-3-propyl-thiourea