Your Location:Home > Products > Medical intermediate > N4-Acetylcytosine
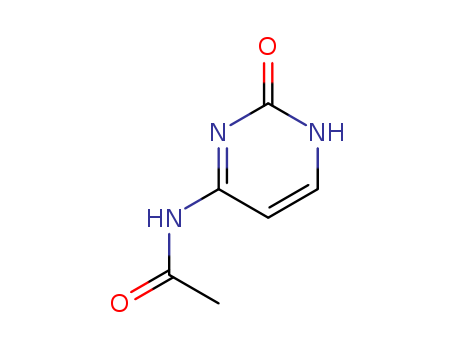

CasNo: 14631-20-0
MF: C6H7N3O2
Packing: 25kg/drum or as required by customer
ynonyms: N4-Acetylcytosine;
Molecular Formula: C6H7N3O2
Molecular Weight: 153.14
CAS Number:14631-20-0
|
Item |
Specification |
|
Appearance |
White to Off-White powder |
|
Loss on drying,% |
≤0.5 |
|
Residue on ignition,% |
≤0.10 |
|
Assay (HPLC),% |
≥98.0 |
|
Acetic acid,% |
≤0.5 |
|
Other single impurities,% |
≤0.5 |
|
total impurities,% |
≤1.0 |
Characteristics: White to Off-White powder. It is slightly soluble in acetone, methanol and dimethyl sulfoxide and insoluble in water.
Used for the synthesis of pharmaceutical intermediates, raw materials of photoreceptors, pyrimidine anti-tumor drugs and anti-AIDS drugs.
Storage: Store in a tightly closed container. Maintain in a cool and dry area.
Package: 25kg/drum or as required by customer.
Expiration Date: 2 years
We synthesized chemically well-defined b...
-
Reaction of monosaccharide aldoses with ...
The cystic fibrosis transmembrane conduc...
The synthesis of glycopyranosyl nucleosi...
The invention discloses a preparation me...
Through systematical comparison of vario...
Two novel series of N-sulfonylamidino py...
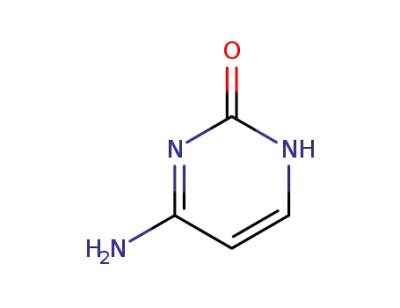
Cytosine

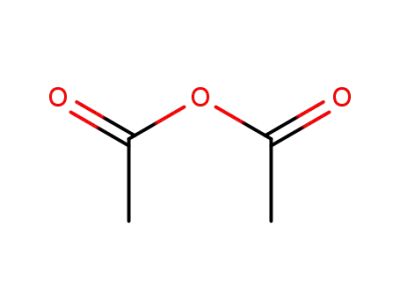
acetic anhydride

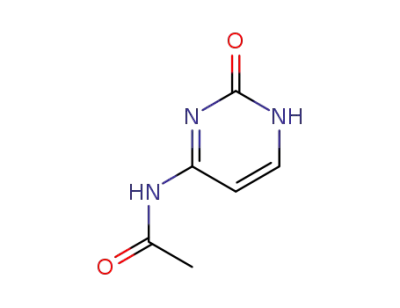
4-N-Acetylcytosine
| Conditions | Yield |
|---|---|
|
With phosphoric acid; at 100 ℃; for 8h;
|
98% |
|
With pyridine; for 2.5h; Heating;
|
97.2% |
|
With pyridine; at 20 ℃;
|
95% |
|
With pyridine; at 20 ℃; for 24h;
|
85% |
|
In N,N-dimethyl-formamide; for 24h; Ambient temperature;
|
78% |
|
for 4h; Heating;
|
75% |
|
|
|
|
In N,N-dimethyl-formamide;
|
|
|
With pyridine;
|
|
|
In pyridine;
|
|
|
Heating;
|
|
|
With pyridine; dmap; In toluene; at 25 - 55 ℃; for 7h;
|
|
|
With pyridine; dmap; In toluene; at 25 - 55 ℃; for 7h;
|
|
|
With pyridine;
|
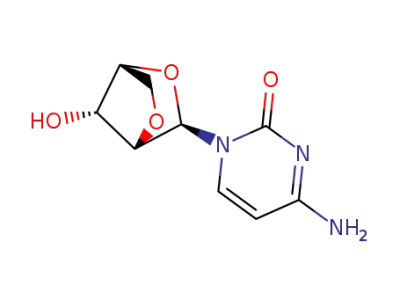
4-Amino-1-(2,5-anhydro-β-D-arabinofuranosyl)pyrimidin-2(1H)-one


4-N-Acetylcytosine

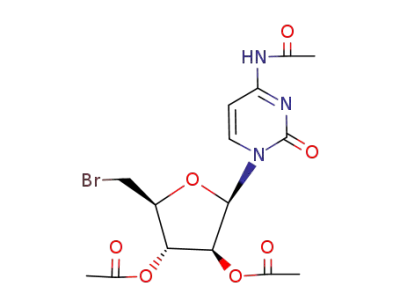
4-Acetamido-1-(2,3-di-O-acetyl-5-bromo-5-deoxy-β-D-arabinofuranosyl)pyrimidin-2(1H)-one

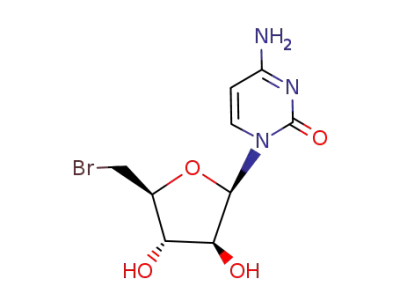
4-Amino-1-(5-bromo-5-deoxy-β-D-arabinofuranosyl)pyrimidin-2(1H)-one
| Conditions | Yield |
|---|---|
|
With hydrogen bromide; In N,N-dimethyl-formamide; at 120 ℃; for 0.416667h;
|
20% 3% 45% |

Cytosine

acetic anhydride
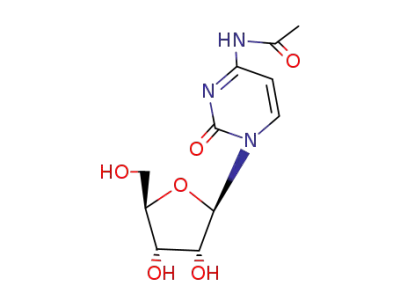
N-acetylcytidine

4-Amino-1-(2,5-anhydro-β-D-arabinofuranosyl)pyrimidin-2(1H)-one
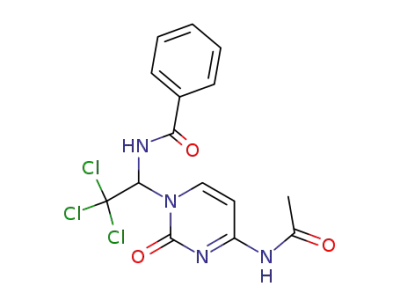
N-[1-(4-Acetylamino-2-oxo-2H-pyrimidin-1-yl)-2,2,2-trichloro-ethyl]-benzamide
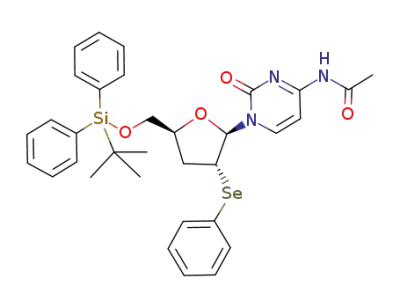
N4-acetyl-1-<5-O-(tert-butyldiphenylsilyl)-3-deoxy-2-Se-phenyl-2-seleno-β-D-erythro-pentofuranosyl>cytosine
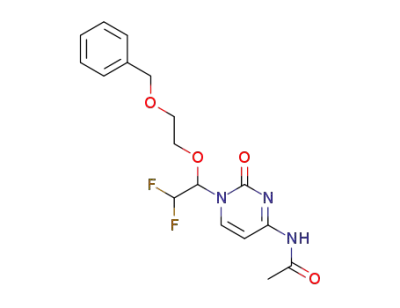
N4-ACETYL-1-[2,2-DIFLUORO-1-[2-(BENZYLOXY)ETHOXY]ETHYL]CYTOSINE
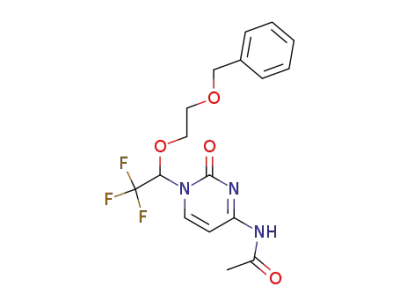
N4-Acetyl-1-[2,2,2-trifluoro-1-[2-(benzyloxy)ethoxy]ethyl]cytosine