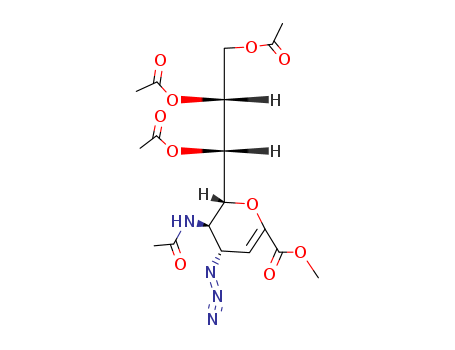

CasNo: 130525-58-5
MF: C18H24N4O10
Appearance: Off-white solid
InChI:InChI=1/C18H24N4O10/c1-8(23)20-15-12(21-22-19)6-13(18(27)28-5)32-17(15)16(31-11(4)26)14(30-10(3)25)7-29-9(2)24/h6,12,14-17H,7H2,1-5H3,(H,20,23)/t12-,14+,15+,16+,17+/m0/s1
Battling the flu: Zanamivir (Relenza) is...
-
Disclosed herein is a small molecule tar...
The present invention provides N-acetyl ...
Sialic acid derivatives, analogs, and th...
The invention relates to fatty acid anti...
![methyl (3aS,4R,7aR)-4-[(1S,2R)-1,2,3-triacetoxy-propyl]-2-methyl-3a,7a-dihydro-4H-pyrano[3,4-d][1,3]oxazole-6-carboxylate](/upload/2026/1/e28563b2-096b-4a58-ae19-9bee32a7a378.png)
methyl (3aS,4R,7aR)-4-[(1S,2R)-1,2,3-triacetoxy-propyl]-2-methyl-3a,7a-dihydro-4H-pyrano[3,4-d][1,3]oxazole-6-carboxylate

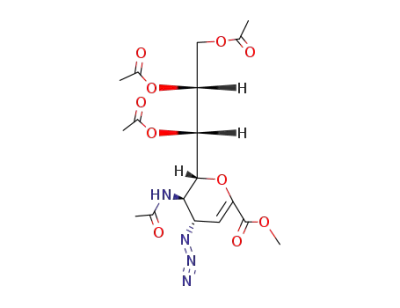
methyl 5-acetamido-4-azido-6-(1,2,3-triacetoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylate
| Conditions | Yield |
|---|---|
|
With
trimethylsilylazide;
In
tert-butyl alcohol;
for 10h;
Inert atmosphere;
Reflux;
|
96% |
|
methyl (3aS,4R,7aR)-4-[(1S,2R)-1,2,3-triacetoxy-propyl]-2-methyl-3a,7a-dihydro-4H-pyrano[3,4-d][1,3]oxazole-6-carboxylate;
With
trimethylsilylazide;
In
tert-butyl alcohol;
for 10h;
Inert atmosphere;
Reflux;
With
hydrogenchloride; sodium nitrite;
In
water; ethyl acetate; tert-butyl alcohol;
at 20 ℃;
for 0.5h;
|
96% |
|
methyl (3aS,4R,7aR)-4-[(1S,2R)-1,2,3-triacetoxy-propyl]-2-methyl-3a,7a-dihydro-4H-pyrano[3,4-d][1,3]oxazole-6-carboxylate;
With
trimethylsilylazide;
In
tert-butyl alcohol;
for 10h;
Inert atmosphere;
Reflux;
With
hydrogenchloride; sodium nitrite;
In
water;
|
96% |
|
With
trimethylsilylazide;
In
tert-butyl alcohol;
for 10h;
Reflux;
Inert atmosphere;
|
84% |
|
With
trimethylsilylazide;
In
tert-butyl alcohol;
at 80 ℃;
for 4h;
|
82.5% |
|
With
trimethylsilylazide;
In
tert-butyl alcohol;
at 80 ℃;
|
78% |
|
With
trimethylsilylazide;
In
tert-butyl alcohol;
for 10.5h;
Heating;
|
76% |
|
With
trimethylsilylazide;
In
tert-butyl alcohol;
at 80 ℃;
for 12h;
Inert atmosphere;
|
73% |
|
With
trimethylsilylazide;
In
tert-butyl alcohol;
at 37 ℃;
for 11.5h;
|
60% |
|
Multi-step reaction with 2 steps
1: 50percent aq. AcOH / ethyl acetate / Ambient temperature
2: 87 percent / diphenylphosphoryl azide, 1,8-diazabicyclo<5.4.0>undec-7-ene / 23 h / Ambient temperature
With
diphenylphosphoranyl azide; acetic acid; 1,8-diazabicyclo[5.4.0]undec-7-ene;
In
ethyl acetate;
|
|
|
Multi-step reaction with 2 steps
1: 77 percent / trifluoroacetic acid / tetrahydrofuran / Ambient temperature
2: 67 percent / PPh3, NH3, DEAD / toluene / 14 h / 0 °C
With
ammonia; triphenylphosphine; trifluoroacetic acid; diethylazodicarboxylate;
In
tetrahydrofuran; toluene;
|
|
|
With
trimethylsilylazide;
In
tert-butyl alcohol;
for 9h;
Reflux;
|
|
|
With
trimethylsilylazide;
In
tert-butyl alcohol;
for 9h;
Reflux;
|
|
|
With
trimethylsilylazide;
|
|
|
With
trimethylsilylazide;
In
tert-butyl alcohol;
|
|
|
With
trimethylsilylazide;
In
tert-butyl alcohol;
|
C18H23NO10


methyl 5-acetamido-4-azido-6-(1,2,3-triacetoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylate
| Conditions | Yield |
|---|---|
|
C18H23NO10;
With
trimethylsilylazide;
In
tert-butyl alcohol;
Inert atmosphere;
Reflux;
With
hydrogenchloride; sodium nitrite;
In
water; tert-butyl alcohol;
for 1h;
|
76% |
|
With
trimethylsilylazide;
In
butan-1-ol;
|
|
|
With
trimethylsilylazide;
In
butan-1-ol;
|
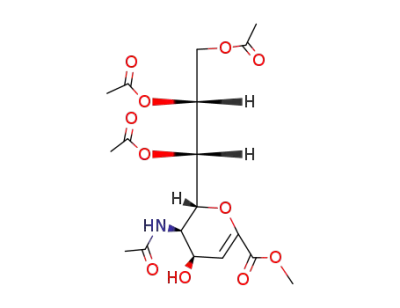
Methyl 5-acetamido-7,8,9-tri-O-acetyl-2,6-anhydro-3,5-dideoxy-D-glycero-D-talo-non-2-enonate
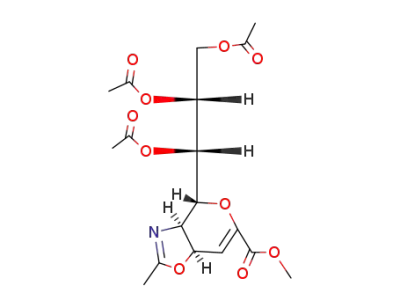
methyl (3aS,4R,7aR)-4-[(1S,2R)-1,2,3-triacetoxy-propyl]-2-methyl-3a,7a-dihydro-4H-pyrano[3,4-d][1,3]oxazole-6-carboxylate
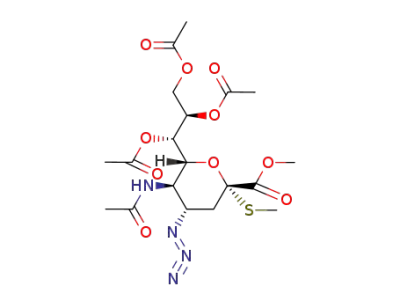
methyl (methyl 5-acetamido-7,8,9-tri-O-acetyl-4-azido-3,4,5-trideoxy-2-thio-D-glycero-α-D-galacto-nonulopyranosid)onate
1,2:3,4-di-O-isopropylidene-α-D-galactopyranose
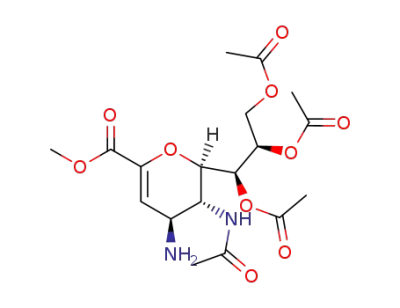
5-acetamido-7,8,9-tri-O-acetyl-2,6-anhydro-4-amino-3,4,5-trideoxy-D-glycero-D-galacto-non-2-enonic acid methyl ester
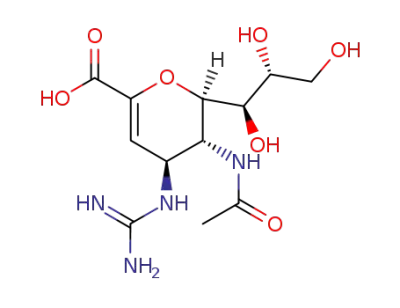
zanamivir
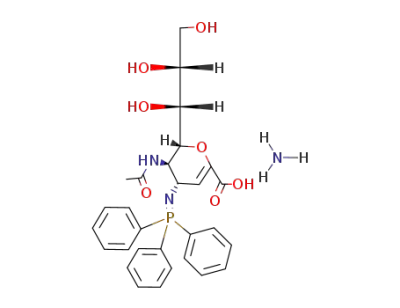
ammonium 5-acetamido-2,3-didehydro-2,3,4,5-tetradeoxy-4-(triphenylphosphoranylidenamino)-D-glycero-D-galacto-2-nonulopyranosidonate
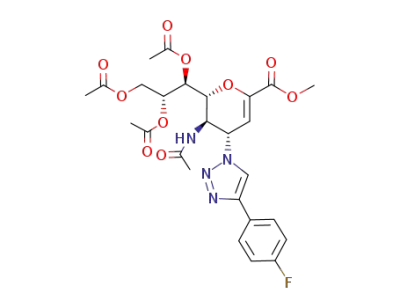
C26H29FN4O10