Your Location:Home > Products > Medical intermediate > Methyl -2,3,4,6-Tetra-O-benzyl-a-D-mannopyranoside
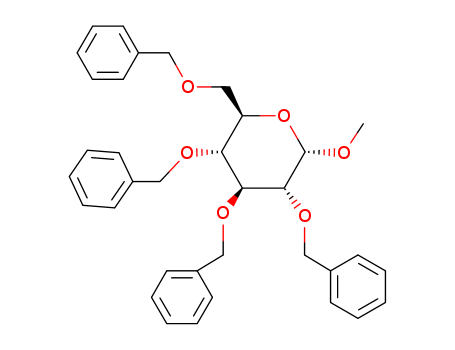

CasNo: 61330-62-9
MF: C35H38O6
Packing: 25kg/drum or according to customer requirements.
Molecular Formula: C35H38O6
Molecular Weight: 554.67
CAS Number:61330-62-9
Specification
|
Item |
Specification |
|
Appearance |
Yellow to wine red viscous liquid |
|
Assay ,% |
≥95.0 |
|
Water,% |
≤0.5 |
|
Residue on ignition,% |
≤0.10 |
Characteristics: It is yellow to wine red viscous liquid. It is easily soluble in butanone and toluene and almost insoluble in water.
Methyl-2,3,4,6-tetra-O-benzyl-D-α-mannopyranoside is an important organic synthetic intermediate. It is used in synthesize for below products:
Storage: Keep tightly closed and store in a dry place.
Package: 25kg/drum or according to customer requirements.
Shelf life: 2 years.
Therapeutic angiogenesis is a potential ...
Presented herein is an improved synthesi...
We have designed unprecedented cholinest...
C-Aryl glycosides are of high value as d...
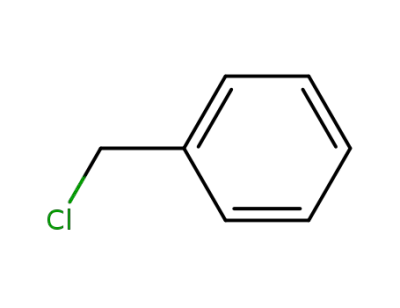
benzyl chloride

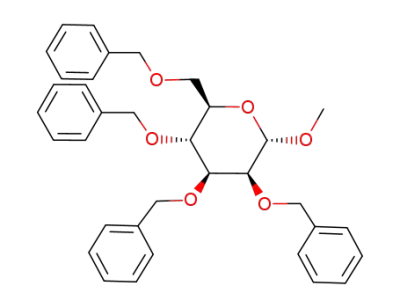
(2R,3R,4S,5S,6S)-3,4,5-tris(benzyloxy)-2-((benzyloxy)methyl)-6-methoxytetrahydro-2H-pyran
| Conditions | Yield |
|---|---|
|
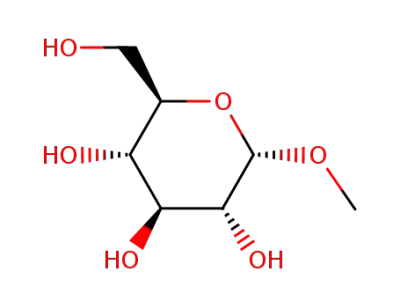
(2R,3S,4S,5S,6S)-2-(hydroxymethyl)-6-methoxytetrahydro-2H-pyran-3,4,5-triol; With sodium hydride; In DMF (N,N-dimethyl-formamide); for 1h;
benzyl chloride; With tetra-(n-butyl)ammonium iodide; In DMF (N,N-dimethyl-formamide); at 20 ℃;
|
71% |

benzyl chloride

methyl-alpha-D-glucopyranoside

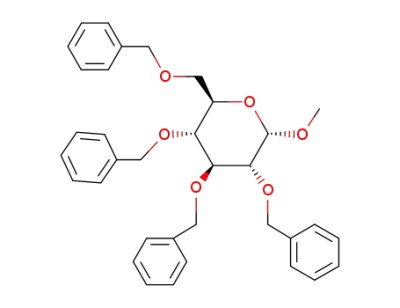
methyl 2,3,4,6-tetra-O-benzyl-α-D-glucopyranoside

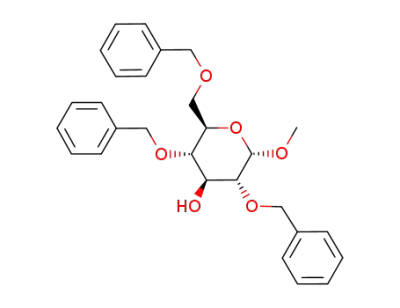
(2R,3S,4S,5R,6S)-3,5-Bis-benzyloxy-2-benzyloxymethyl-6-methoxy-tetrahydro-pyran-4-ol

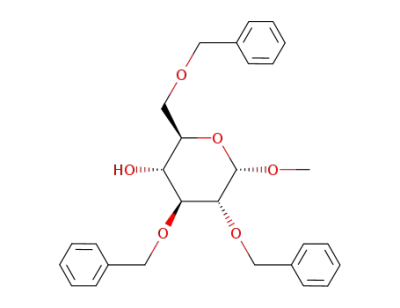
methyl O-2,3,6-tri-O-benzyl-α-D-glucopyranoside
| Conditions | Yield |
|---|---|
|
With sodium hydride; In mineral oil; at 100 ℃; for 5h; Temperature; regioselective reaction; Inert atmosphere;
|
29% 20% |
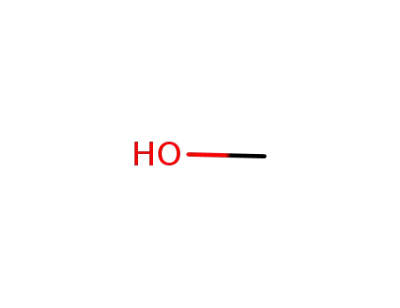
methanol
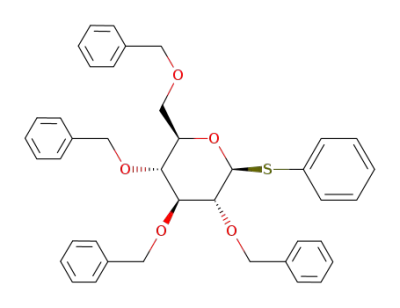
phenyl 2,3,4,5-tetra-O-benzyl-1-thio-β-D-glucopyranoside
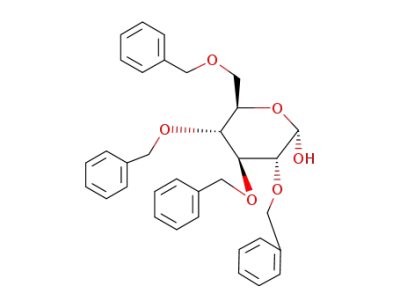
2,3,4,6-tetra-O-benzyl-D-glucopyranose
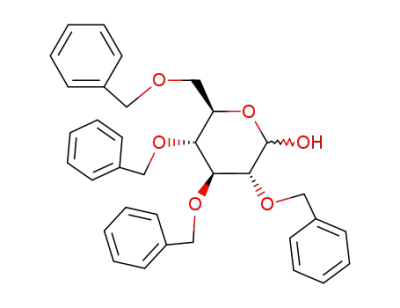
2,3,4,6-Tetra-O-benzyl-D-glucopyranose
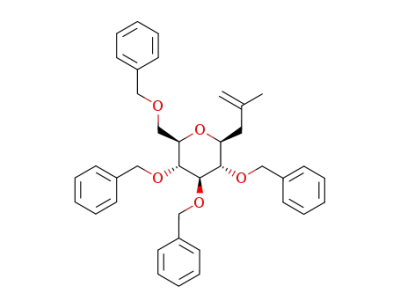
2-methyl-3-(2,3,4,6-tetra-O-benzyl-α-D-glucopyranosyl)propene
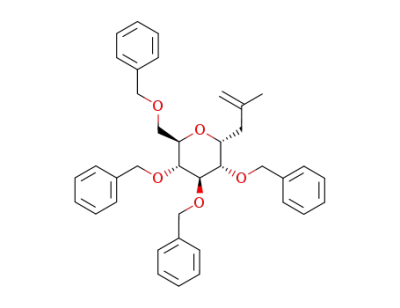
2-methyl-3-(2,3,4,6-tetra-O-benzyl-β-D-glucopyranosyl)propene
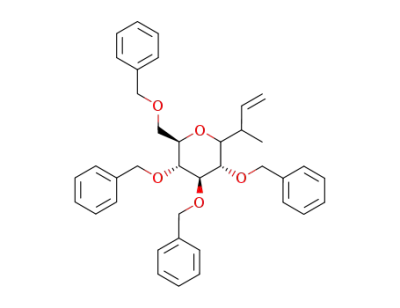
3-(2,3,4,6-tetra-O-benzyl-α-D-glucopyranosyl)butene
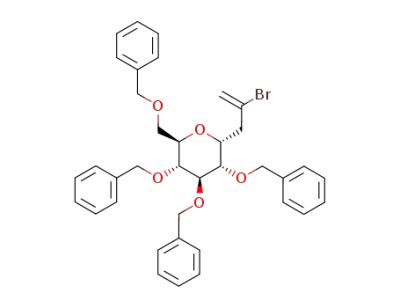
2-bromo-3-(2,3,4,6-tetra-O-benzyl-α-D-glucopyranosyl)propene