Your Location:Home > Products > Medical intermediate > Methyl -2,3,4,6-Tetra-O-benzyl-a-D-Glucopyranoside
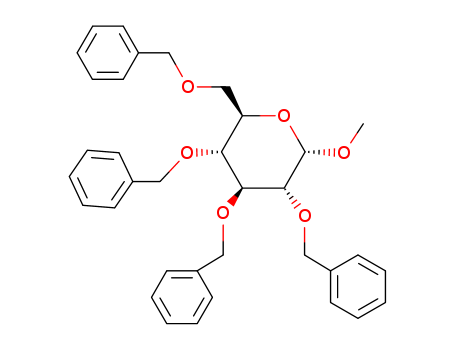

CasNo: 17791-37-6
MF: C35H38O6
Appearance: yellow thick oil
Synonym: Methyl-2,3,4,6-tetrakis-O-(phenylmethyl)-α-D-galactopyranoside
Molecular Formula: C35H38O6
Molecular weight: 554.67
CAS No.: 53008-63-2
Quality Standard:
| Item | Specification |
|---|---|
| Appearance | pale-yellow to red viscous liquid |
| Assay (HPLC), % | ≥90 |
| Water,% | ≤0.5 |
Properties: pale-yellow to red viscous liquid. Dissolved in toluene, acetone and 1,4-dioxane etc.
Storage: Store in a tightly closed container. Store in a cool and dry area.
Package: 25kg /200kg/drum or as required by customer.
Expiration Date: 2 years
Methyl-2,3,4,6-tetra-O-benzyl-α-D-galactopyranoside is an important organic synthetic intermediate. This product will hydrolyze into 2,3,4,6-tetra-O-benzyl-α-D-galactopyranoside in acidic conditions, the hydrolysis product is widely used in the synthesis of polysaccharides, glycoconjugates, molecular probes, drug intermediates and so on.
Reacting with acetic anhydride, it can be converted into 1,6-di-O-acetyl-,2,3,4-tri-O-benzyl-α-D-galactopyranoside and 1-O-acetyl-2,3 4,6-tetra-O-benzyl-α-D-galactopyranoside Undersuitable reaction conditions .
It can be conveniently converted into 2,3,4,6-tetra-O- benzyl-α-D-galactosyl fluoride, chloride,bromide,iodide.
As initial material, it can also be synthesized into phosphonic acid galactosyl transferase inhibitors, the inhibitors can inhibitα-1 and β-1,3-,4- and galactosyl transferase.
Therapeutic angiogenesis is a potential ...
Presented herein is an improved synthesi...
We have designed unprecedented cholinest...
C-Aryl glycosides are of high value as d...
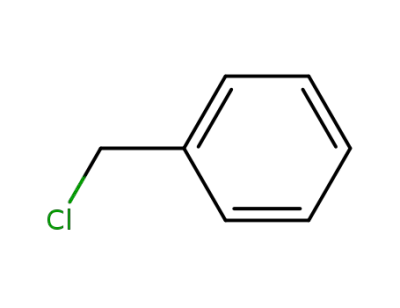
benzyl chloride

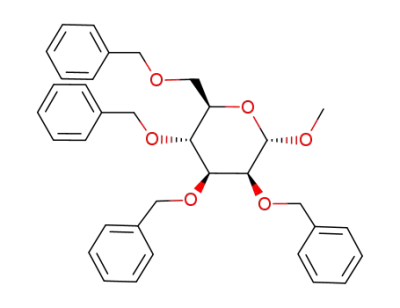
(2R,3R,4S,5S,6S)-3,4,5-tris(benzyloxy)-2-((benzyloxy)methyl)-6-methoxytetrahydro-2H-pyran
| Conditions | Yield |
|---|---|
|
(2R,3S,4S,5S,6S)-2-(hydroxymethyl)-6-methoxytetrahydro-2H-pyran-3,4,5-triol; With sodium hydride; In DMF (N,N-dimethyl-formamide); for 1h;
benzyl chloride; With tetra-(n-butyl)ammonium iodide; In DMF (N,N-dimethyl-formamide); at 20 ℃;
|
71% |

benzyl chloride

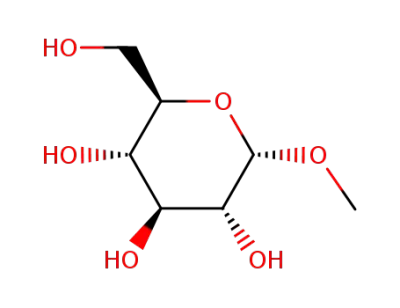
methyl-alpha-D-glucopyranoside

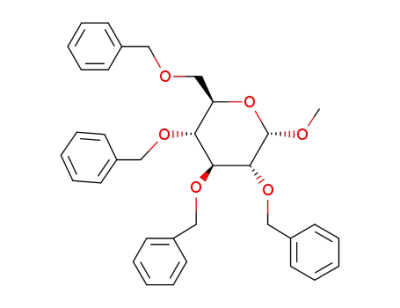
methyl 2,3,4,6-tetra-O-benzyl-α-D-glucopyranoside

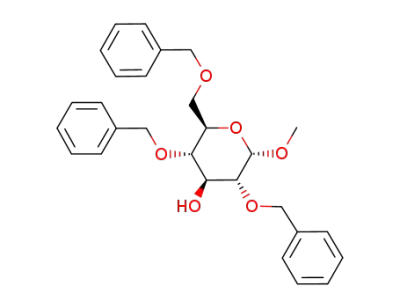
(2R,3S,4S,5R,6S)-3,5-Bis-benzyloxy-2-benzyloxymethyl-6-methoxy-tetrahydro-pyran-4-ol

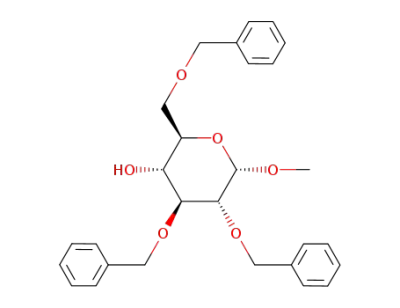
methyl O-2,3,6-tri-O-benzyl-α-D-glucopyranoside
| Conditions | Yield |
|---|---|
|
With sodium hydride; In mineral oil; at 100 ℃; for 5h; Temperature; regioselective reaction; Inert atmosphere;
|
29% 20% |
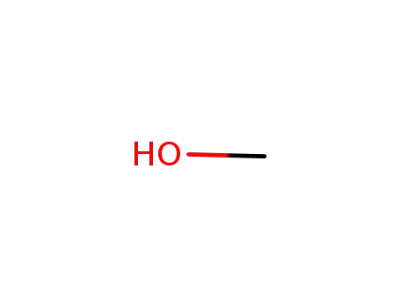
methanol
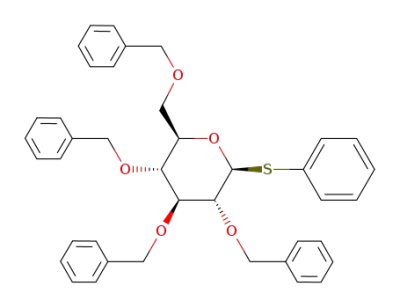
phenyl 2,3,4,5-tetra-O-benzyl-1-thio-β-D-glucopyranoside
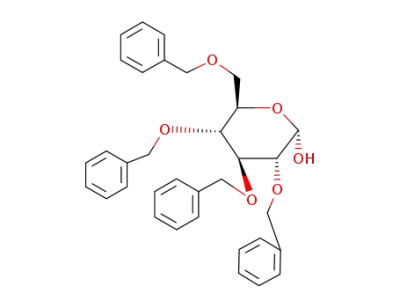
2,3,4,6-tetra-O-benzyl-D-glucopyranose
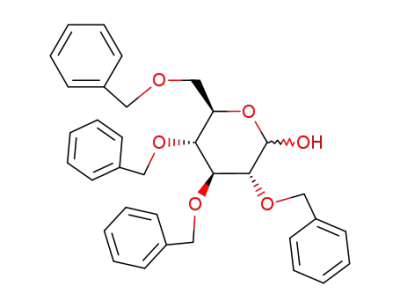
2,3,4,6-Tetra-O-benzyl-D-glucopyranose
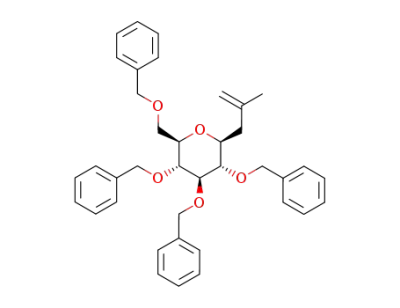
2-methyl-3-(2,3,4,6-tetra-O-benzyl-α-D-glucopyranosyl)propene
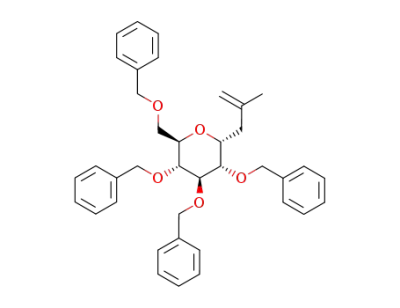
2-methyl-3-(2,3,4,6-tetra-O-benzyl-β-D-glucopyranosyl)propene
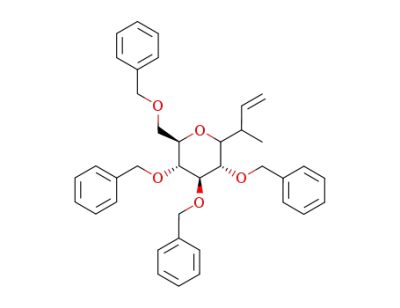
3-(2,3,4,6-tetra-O-benzyl-α-D-glucopyranosyl)butene
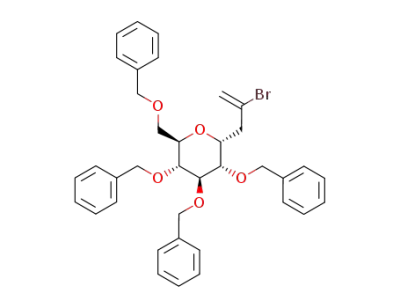
2-bromo-3-(2,3,4,6-tetra-O-benzyl-α-D-glucopyranosyl)propene