Your Location:Home > Products > Medical intermediate > Methyl 4,6-O-Benzylidene-α-D-Galactopyranoside
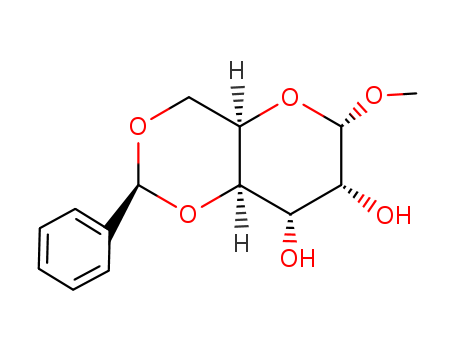

CasNo: 72904-85-9
MF: C14H18 O6
Appearance: White to off-white crystalline powder
Purity: ≥98.0
NAME:Methyl 4,6-O-benzylidene-α-D-galactopyranoside
CAS No.:72904-85-9
Quality Standard:
| Item | Specification |
| Appearance | White to off-white crystalline powder |
| Assay , % | ≥98.0 |
| Melting point, ℃ | 168.0-175.0 |
| Loss on drying, % | ≤1.0 |
| Residue on ignition, % | ≤0.10 |
Methyl-4, 6-benzylidene -α -D-galactopyranoside is a commonly used benzylidene protected carbohydrate derivative, usually as an intermediate, widely used in the synthesis of a variety of compounds.
The benzylidene acetal group is one of t...
The first known report on the fluoride c...
Multivalent carbohydrate-lectin interact...
The crystal structure of methyl β-d-mann...
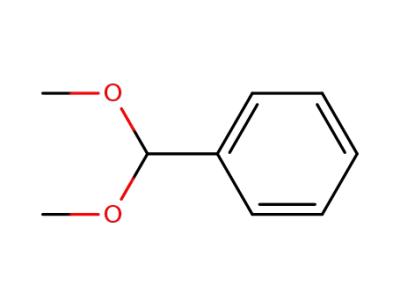
benzaldehyde dimethyl acetal

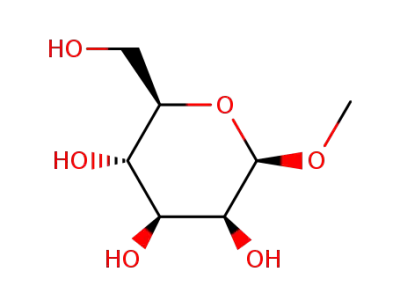
Methyl mannoside

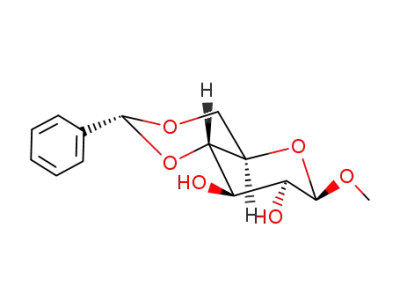
Methyl-4,6-O-(phenylmethylene)-β-D-mannopyranoside
| Conditions | Yield |
|---|---|
|
With toluene-4-sulfonic acid; In N,N-dimethyl-formamide; at 20 ℃; for 48h;
|
65% |
|
With toluene-4-sulfonic acid; In N,N-dimethyl-formamide; at 20 ℃;
|
61% |

benzaldehyde dimethyl acetal

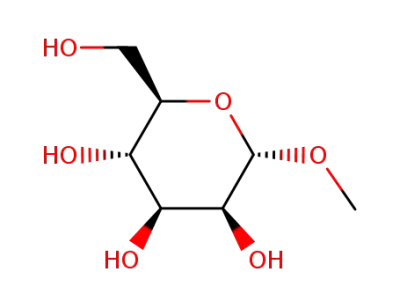
(2R,3S,4S,5S,6S)-2-(hydroxymethyl)-6-methoxytetrahydro-2H-pyran-3,4,5-triol

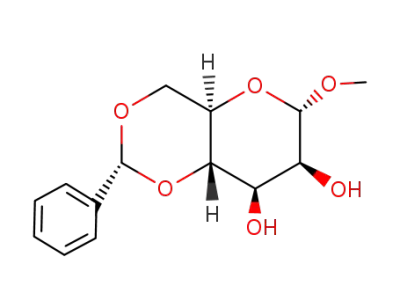
methyl-4,6-O-phenylmethylene-α-D-mannopyranoside
| Conditions | Yield |
|---|---|
|
With camphor-10-sulfonic acid;
|
98% |
|
With 1,3,5-trichloro-2,4,6-triazine; In acetonitrile; at 60 ℃; for 0.166667h; regioselective reaction; Sonication; Inert atmosphere;
|
98% |
|
With toluene-4-sulfonic acid; 1-butyl-3-methylimidazolium Tetrafluoroborate; at 80 ℃; for 2h;
|
88% |
|
With 3,4-bis((3,5-bis(trifluoromethyl)phenyl)amino)cyclobut-3-ene-1,2-dione; In acetonitrile; at 20 ℃; for 2.5h; Inert atmosphere;
|
86% |
|
With toluene-4-sulfonic acid; In N,N-dimethyl-formamide; at 50 - 70 ℃; for 4h;
|
78% |
|
With toluene-4-sulfonic acid; In N,N-dimethyl-formamide; acetonitrile;
|
77% |
|
With perchloric acid on silica gel; In N,N-dimethyl-formamide; at 20 ℃;
|
73% |
|
With toluene-4-sulfonic acid; In N,N-dimethyl-formamide; at 60 ℃; for 1h; Inert atmosphere;
|
70% |
|
With toluene-4-sulfonic acid; In N,N-dimethyl-formamide; at 60 ℃; for 1h;
|
70% |
|
With toluene-4-sulfonic acid; In N,N-dimethyl-formamide; at 60 ℃; for 1h;
|
70% |
|
With toluene-4-sulfonic acid; In N,N-dimethyl-formamide; at 60 ℃; for 2h; under 150.015 Torr;
|
52% |
|
With camphor-10-sulfonic acid; In N,N-dimethyl-formamide; at 20 ℃; for 24h;
|
48% |
|
With camphor-10-sulfonic acid; In N,N-dimethyl-formamide; at 60 ℃; for 32.75h; under 195.016 Torr;
|
37% |
|
With toluene-4-sulfonic acid; In N,N-dimethyl-formamide; at 60 ℃; for 6h; under 150.015 Torr;
|
31% |
|
With camphor-10-sulfonic acid; In N,N-dimethyl-formamide; at 20 ℃;
|
|
|
In N,N-dimethyl-formamide; at 40 ℃; for 4h;
|
12 g |
|
With iodine; In acetonitrile; at 20 ℃; for 3h;
|
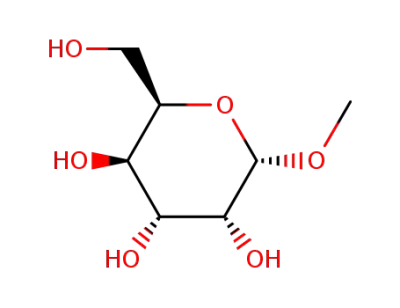
methyl β-D-galactopyranoside

benzaldehyde dimethyl acetal

(2R,3S,4S,5S,6S)-2-(hydroxymethyl)-6-methoxytetrahydro-2H-pyran-3,4,5-triol
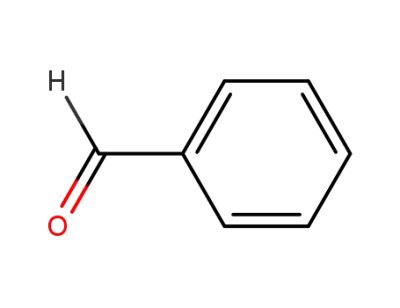
benzaldehyde
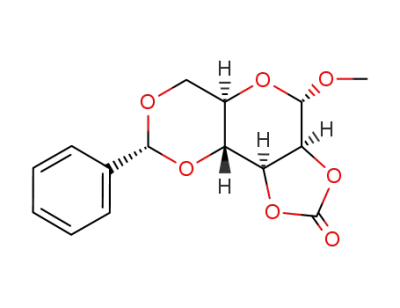
Methyl-4,5-O-benzyliden-2,3-carbonato-α-D-mannopyranosid
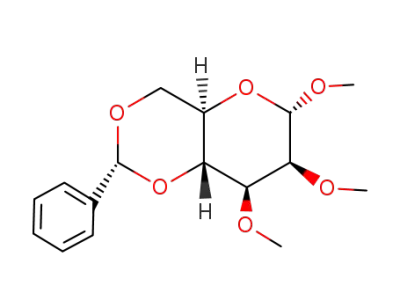
methyl 4,6-O-benzylidene-2,3-di-O-methyl-α-D-mannopyranoside
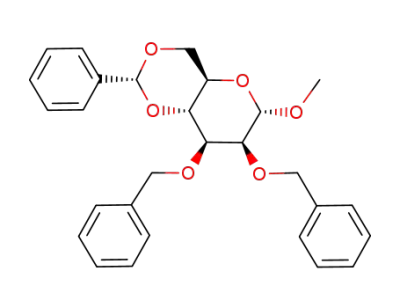
methyl 2,3-di-O-benzyl-4,6-O-benzylidene-α-D-mannopyranoside
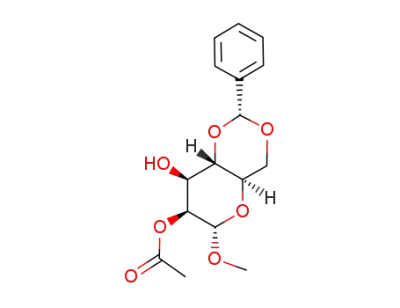
Methyl 2-O-acetyl-4,6-O-(phenylmethylene)-α-D-mannopyranoside