Your Location:Home > Products > Medical intermediate > Monoacetone-D-Glucose
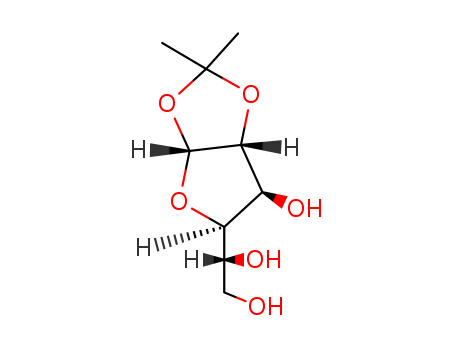

CasNo: 18549-40-1
MF: C9H16O6
Appearance: white crystalline solid
Packing: 25kgs/drum or as required by the customer.
Synonyms: Acetone Glucose, Acetone-D-Glucose, 1,2-O-Isopropylidene-α-D-glucofuranose, 1,2-O-Isopropylidene-alpha-D-glucofuranose, 1,2-O-(1-methylethylidene)-α-D-glucofuranose, Monoacetone Glucofuranose.
Molecular Formula: C9H16O6
Molecular weight: 220.22
CAS No.: 18549-40-1
EC No.: 242-420-9
Quality Standard:
|
Item |
Specification |
|
Appearance Assay (GC), % Melting point, ℃ Specific rotation, Loss on drying, % Residue on ignition, % Heavy metals, ppm TLC |
White microcrystalline solid ≥98 158~162
-10~ -14 ≤1.0 ≤0.2 ≤10 Single spot |
Properties: It is white microcrystalline solid. Dissolve in water, acetone, ethanol, tetrahydrofuran, dimethyl formamide etc.
This product is an important pharmaceutical intermediates. Used in synthesis Tribenoside,synthesis nojirimycin and deoxynojirimycin for treatment diabetes, complexes that carbohydrate combine with organic metal including platinum, osmium tetroxide, ruthenium, etc for treatment cancer, other anticancer or antitumor pharmaceuticals and their precursor such as organic phosphide, and azole that contain glycosyl for resistance to junin virus and fever virus such as dengue fever, dengue hemorrhagic fever and Argentine hemorrhagic fever.
Storage: Store in a tightly closed container.Store in a cool,dry area.
Package: 25kgs/drum or as required by the customer.
Expiration Date: 2 years.
Acetonides undergo chemoselective deprot...
The intramolecular Diels-Alder (IMDA) re...
-
The site-specific natural hydrogen isoto...
-
-
-
(1S,2R,5R,7S)-2-Hydroxy-exo-brevicomin e...
Stable isotopes in tree rings are import...
-
Treatment of D-glucitol 1,3:2,4:5,6-tris...
-
Glycoconjugation to target the Warburg e...
Here, we report a highly selective, effi...
An efficient cleavage-isopropylidenation...
-
-
Using the “chiral pool” approach, two mo...
Organic reactions in the aqueous environ...
The reaction of t-butylmagnesium chlorid...
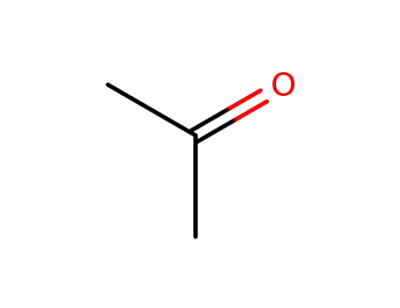
acetone

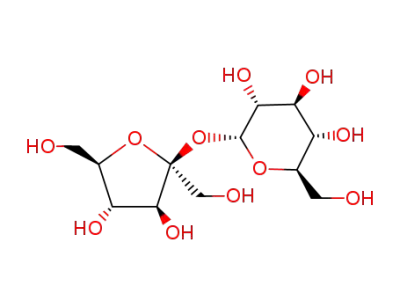
Sucrose

2,3;4,5-di-O-isopropylidene-β-D-fructopyranose

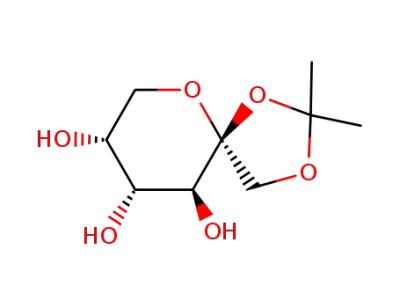
1,2-O-isopropylidene-β-D-fructopyranose

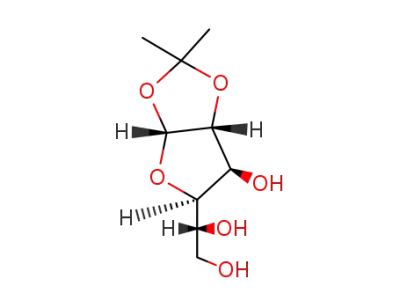
1,2-O-isopropylidene-α-D-glucofuranose
| Conditions | Yield |
|---|---|
|
With acetic acid; iodine; Yield given. Multistep reaction. Yields of byproduct given; 1) reflux, 4 h, 2) room temp., 22 h;
|
|
|
With acetic acid; iodine; Yield given. Multistep reaction. Yields of byproduct given; 1) reflux, 4 h, 2) room temp., 22 h;
|

acetone


Sucrose


2,3;4,5-di-O-isopropylidene-β-D-fructopyranose


1,2-O-isopropylidene-α-D-glucofuranose
| Conditions | Yield |
|---|---|
|
acetone; Sucrose; With phosphoric acid; zinc(II) chloride;
With acetic acid;
|
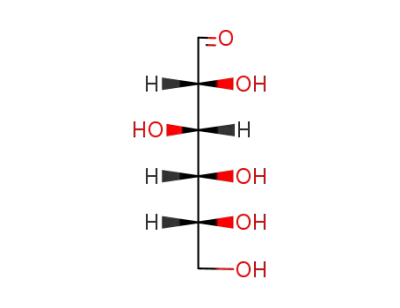
D-glucose

acetone
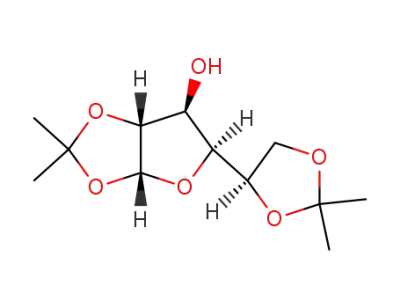
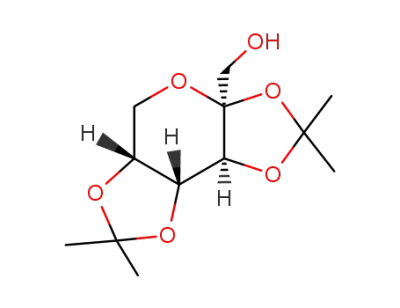
1,2:5,6-di-O-isopropylidene-α-D-glucofuranose
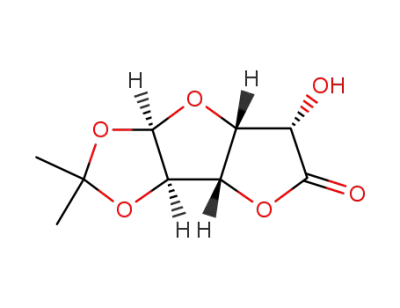
D-glucurono-3,6-lactone acetonide
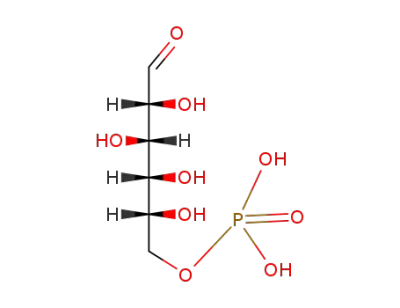
D-glucose 6-phosphate
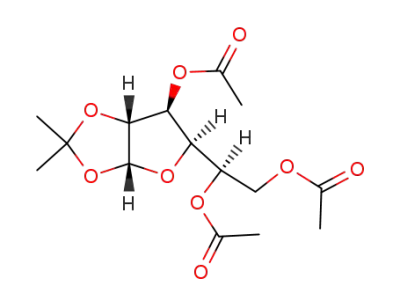
3,5,6-tri-O-acetyl-1,2-O-isopropylidene-α-D-glucofuranose
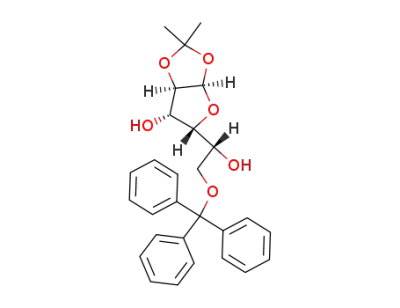
1,2-O-isopropylidene-6-O-trityl-α-D-glucofuranoside
O3,O5-dibenzoyl-O1,O2-isopropylidene-O6-trityl-α-D-glucofuranose