Your Location:Home > Products > Medical intermediate > Diacetone-D-Glucose
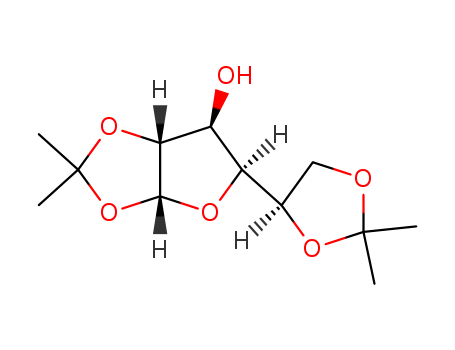

CasNo: 582-52-5
MF: C12H20O6
Appearance: white to light yellow crystal powder
Synonyms: Diacetone-D-glucose;1,2:5,6-Diisopropylidene-D-glucose;D-Glucose diacetonide; Diacetone glucose.
Molecular Formula: C12H20O6
Molecular weight: 260.28
CAS No.: 582-52-5
EC No.: 209-486-0
RTECS: LZ49580000
Quality Standard:
|
Item |
Specification |
|
Appearance |
White crystalline power |
|
Melting Point, ℃ |
107-113 |
|
Assay,% |
≥98 |
|
Specific rotation, |
-17~ -19 |
|
Heavy Metals,ppm |
≤ 10 |
|
Loss on drying,% |
≤1.0 |
|
Residue on ignition,% |
≤0.1 |
|
TLC |
Single spot |
Properties: It's white crystalline powder.It's not flammability.It's innocuity.
It is mainly used in biochemical reaction and used as medicine intermediate. For example cephalosporin and penicilline. It is used in synthesize for below products: L-gulose; 1,2:5,6-Di-O-isopropylidene-a-D-gulofuranose; Alllose; 1,2:5,6-Double-O-Isopropylidene-3-C-(1-Methoxycarbonyl)Ethide-α-D-Furanose); 1,2:5,6-Di-O-isopropylidene-a-D-ribo-hexofuranose-3-ulose;6-deoxy idose; L-acovenose;3-O-Methyl-1,2:5,6-di-O-isopropylidene-a-D- glucofuranose ;3-O-acryloyl-1,2:5,6-di-O-isopropylidene-a-D- glucofuranose;3-O-Methacryloyl-1,2:5,6-di-O-isopropylidene-a-D- glucofuranose; 3-O-Allyl-1,2:5,6-di-O-isopropylidene-a-D- glucofuranose; 3-O-Benzyl-1,2:5,6-di-O-isopropylidene-a-D-glucofuranose; 3-O-Acetyl-1,2:5,6-di-O-isopropylidene-a-D-glucofuranose; 3-O-[2-Dimethylaminoethyl]-1,2:5,6-bis-O-1-methylethylidene-a-D-glucofuranose; 1,2:5,6-Di-O-isopropylidene-3-O-methylsulfonyl-alpha-D-glucofuranose; 3-Deoxy-1,2:5,6-di-O-isopropylidene-D-glucose
Storage: Store in a tightly closed container. Store in a cool and dry area.
Package: 10kgs/drum or as required by the customer.
A new, direct, and diastereoselective sy...
Dermatan sulfate (DS) is composed of a r...
1,2-O-Isopropylidene-3-O-3(N',N'-dimethy...
A method for making an optically active ...
The cleavage of benzyl ethers by catalyt...
Boron neutron capture therapy (BNCT) is ...
A synthesis method of a release type xyl...
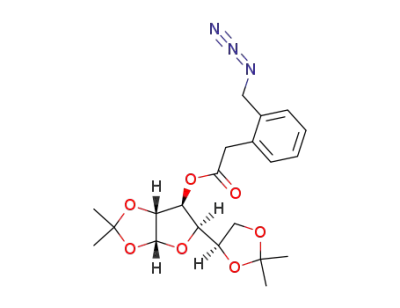
3-O-(2-azidomethyl)phenylacetyl-1,2:5,6-di-O-isopropylidene-α-D-glucofuranose

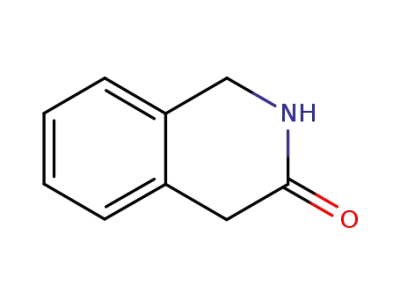
1,4-dihydro-3(2H)-isoquinolinone

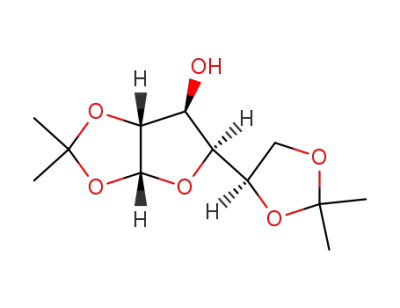
1,2:5,6-di-O-isopropylidene-α-D-glucofuranose
| Conditions | Yield |
|---|---|
|
With hydrogen; Lindlar's catalyst; In methanol; at 20 ℃; for 3h;
|
100% |
![(3aR,5R,6S,6aR)-6-(3,5-Dimethoxy-benzyloxy)-5-((R)-2,2-dimethyl-[1,3]dioxolan-4-yl)-2,2-dimethyl-tetrahydro-furo[2,3-d][1,3]dioxole](/upload/2026/1/78b27cf2-ac3c-4f1e-9d0d-8571d3c08929.png)
(3aR,5R,6S,6aR)-6-(3,5-Dimethoxy-benzyloxy)-5-((R)-2,2-dimethyl-[1,3]dioxolan-4-yl)-2,2-dimethyl-tetrahydro-furo[2,3-d][1,3]dioxole

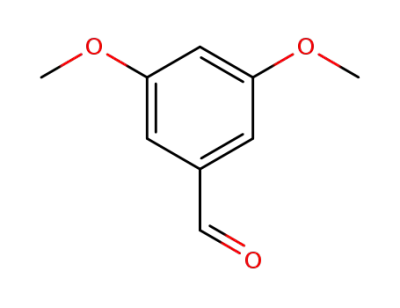
3,5-dimethoxybenzaldehdye


1,2:5,6-di-O-isopropylidene-α-D-glucofuranose
| Conditions | Yield |
|---|---|
|
With 2,3-dicyano-5,6-dichloro-p-benzoquinone; In dichloromethane; water; for 2.5h; Ambient temperature;
|
86% |
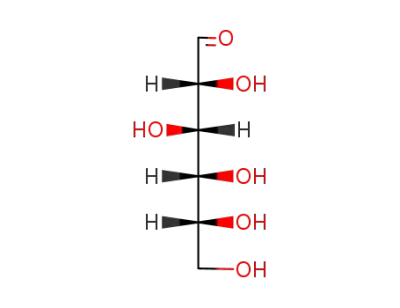
D-glucose
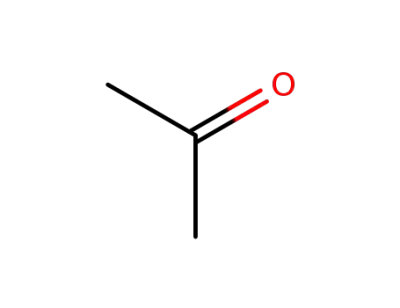
acetone
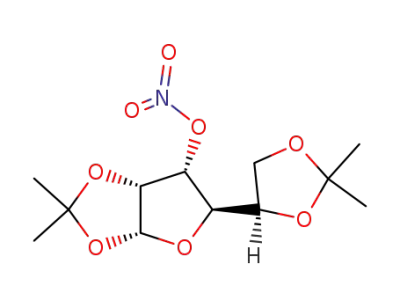
(3aR,5R,6R,6aR)-5-((R)-2,2-Dimethyl-[1,3]dioxolan-4-yl)-2,2-dimethyl-6-nitrooxy-tetrahydro-furo[2,3-d][1,3]dioxole
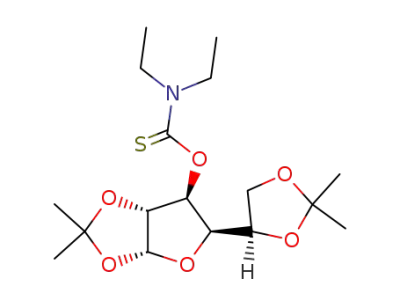
Diethyl-thiocarbamic acid O-[(3aR,5R,6S,6aR)-5-((R)-2,2-dimethyl-[1,3]dioxolan-4-yl)-2,2-dimethyl-tetrahydro-furo[2,3-d][1,3]dioxol-6-yl] ester
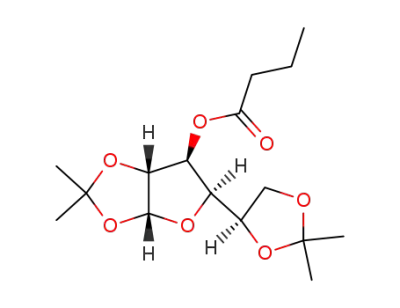
O3-butyryl-O1,O2;O5,O6-diisopropylidene-α-D-glucofuranose
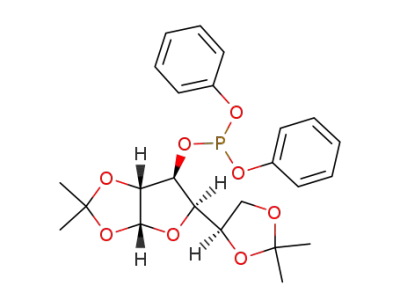
O3-diphenoxyphosphino-O1,O2;O5,O6-diisopropylidene-α-D-glucofuranose
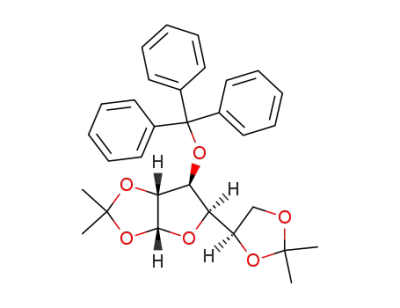
1,2:5,6-di-O-isopropylidene-3-O-trityl-α-D-glucofuranose
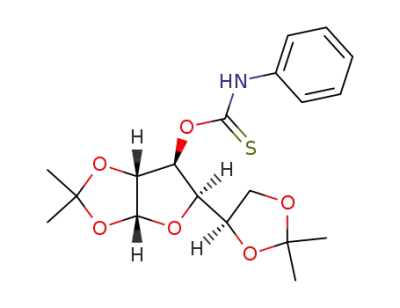
1,2:5,6-di-O-isopropylidene-3-(N-phenylthioxocarbamoyl)-α-D-glucofuranose