Your Location:Home > Products > Medical intermediate > 1,2-O-Isopropylidene-α-D-Xylofuranose
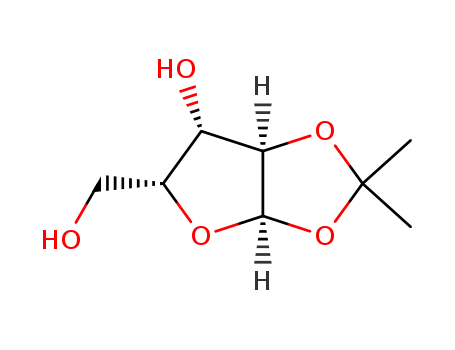

CasNo: 20031-21-4
MF: C8H14O5
Appearance: White crystalline solid
Packing: 25kg/drum or as required by customer.
Purity: ≥98.0
Synonyms: Monoacetone-D-xylose;1,2-monoacetone-D-xylofuranose; 1-O,2-O-Isopropylidene-α-D-xylofuranose; 1,2-O-(1-methylethylidene)-α-D-xylofuranose
Molecular Formula: C8H14O5
Molecular weight: 190.19
CAS No.: 20031-21-4
EC No.: 606-426-9
Molecular structure:
Quality Standard:
|
Item |
Specification |
|
Appearance Assay(GC) , % Specific rotation, |
White to light yellow powder ≥98.0 -17.0~-21.0
|
Characteristics: White to light yellow powder. Dissolve in water, chlorform, ethyl acetate, methanol etc.
Monoacetone-D-xylose is an important raw material and intermediate of pharmaceutical. For example, it is used for preparation pharmaceuticals such as derivatives of enamine/cyclic enamine and vitamin D3, chiral medicines, S-ribosylhomocysteinase, antigen-4, anti-mycobacterium tuberculosis or anti-tuberculosis medicines with no drug-resistant, and other pharmaceuticals of anti-viral, bacteria, cancer. It used in synthesis derivatives of thiosugar which indicate potent anti-cancer, AIDS virus and antithrombotic activity. Synthesis derivatives of tetrahydrofuran for treatment AIDS, herpes,ocular disease such as glaucoma and hypertension ocular, virus of vaccinia and cytomegalo, and DNA disease. Synthesis derivatives of cyclohexanehexol for treatment ocular and neurodegeneratie diseases,derivatives of oxetane δ-Amino Acids,pharmaceutical for diabetes,Oseltamivir phosphate (Tamiflu) for treatment influenza.
In the area of pharmaceutical design and development, it is used in synthesis analogues, mimetics or derivatives of RNA and nucleoside.
It is used as catalyst and chiral auxiliary in asymmetric synthesis.
It is used in the biochemistry and molecular biology for synthesis isoprenoid.
It is used in agriculture and forestry for synthesis herbicides and plant growth regulator.
It is used in carbohydrate synthesis for preparation furanose, arabifuranose, erythrose, threose and other derivatives of xylose etc.
In the food industry, it is used in preparation food surfactant such as monosaccharide fatty acid esters.
In material science field, it is used in synthesis chiral block and surgical applications polymer which can bioerodable.
In the RNA and DNA study, it is used for synthesis nucleosides and xylosides which is labeled by isotopes of hydrogen.
Storage: Store in a tightly closed container. Maintain in a cool and dry area.
Package: 25kg/drum or as required by customer.
Expiration Date: 2 years
A mild and efficient strategy for the co...
-
-
Acetonides undergo chemoselective deprot...
The present disclosure relates to, among...
A series of novel α-L-threose nucleoside...
Kinetoplastid parasites are the causativ...
Manipulating the stereochemistry of poly...
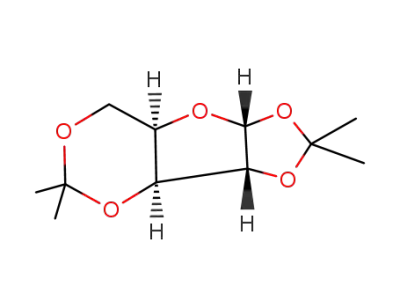
1,2:3,5-di-O-isopropylidene-D-xylofuranose

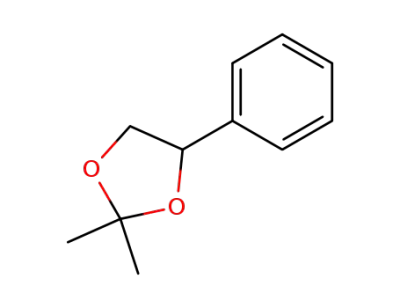
2,2-dimethyl-4-phenyl-1,3-dioxolane

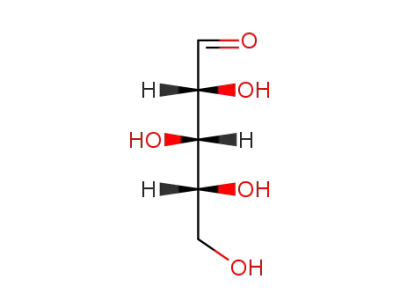
D-xylose

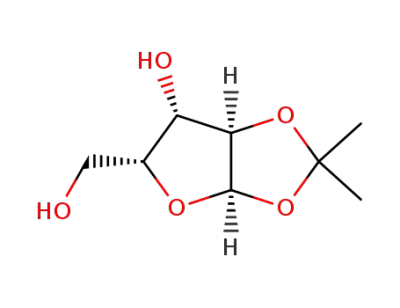
1,2-O-isopropylidene-α-D-xylose
| Conditions | Yield |
|---|---|
|
With phenylethane 1,2-diol; toluene-4-sulfonic acid; In dichloromethane; for 19h;
|
32% 50% |
|
With phenylethane 1,2-diol; toluene-4-sulfonic acid; In dichloromethane; for 19h;
|
50 % Chromat. 32 % Chromat. |
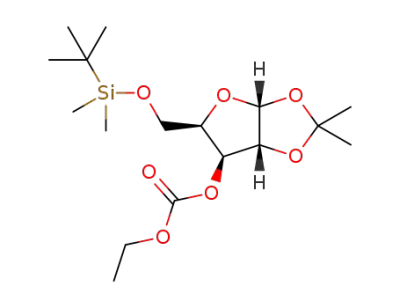
5-O-tert-butyldimethylsilyl-1,2-O-isopropylidene-3-O-ethoxycarbonyl-α-D-xylofuranoside

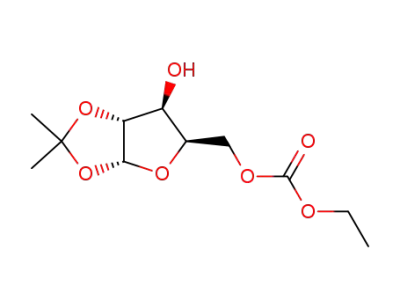
1,2-O-isopropylidene-5-O-ethoxycarbonyl-α-D-xylofuranoside


1,2-O-isopropylidene-α-D-xylose
| Conditions | Yield |
|---|---|
|
With tetrabutyl ammonium fluoride; In tetrahydrofuran; at 20 ℃; for 0.166667h;
|
92% 4% |
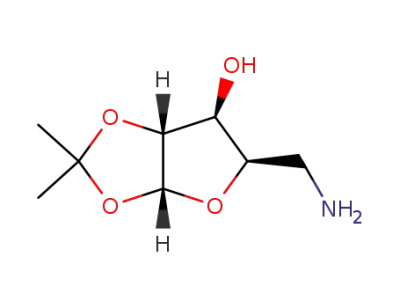
5-amino-5-deoxy-1,2-O-isopropylidene-a-D-xylofuranose

1,2:3,5-di-O-isopropylidene-D-xylofuranose
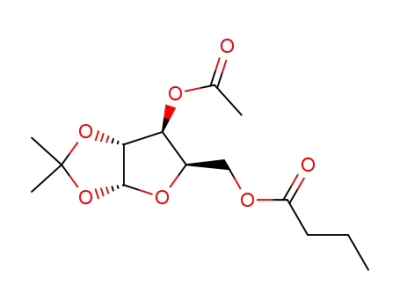
Butyric acid (3aR,5R,6S,6aR)-6-acetoxy-2,2-dimethyl-tetrahydro-furo[2,3-d][1,3]dioxol-5-ylmethyl ester
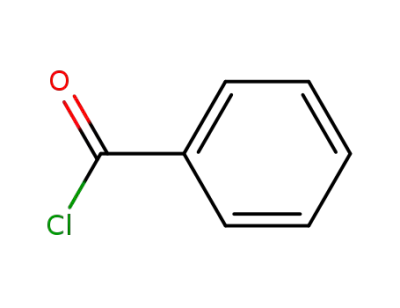
benzoyl chloride
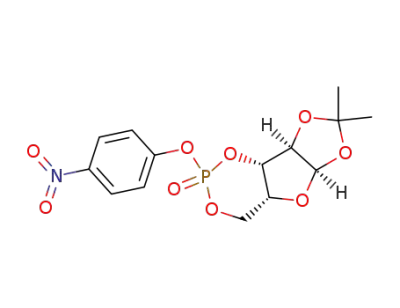
O1,O2-isopropylidene-O3,O5-(4-nitro-phenoxyphosphoryl)-α-D-xylofuranose

D-xylose
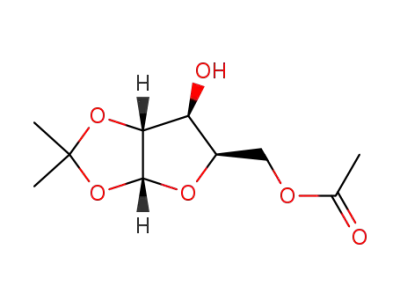
5-O-acetyl-1,2-O-isopropylidene-α-D-xylofuranose
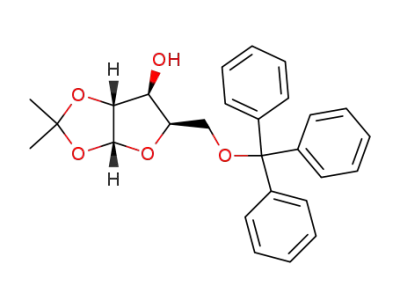
1,2-O-isopropylidene-5-O-trityl-α-D-xylofuranose