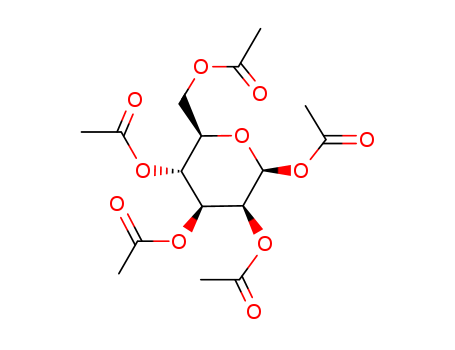
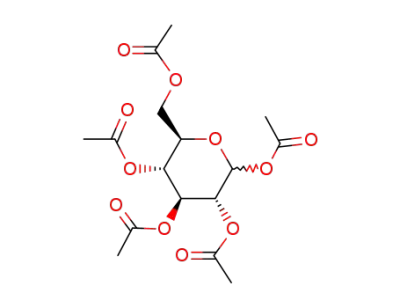
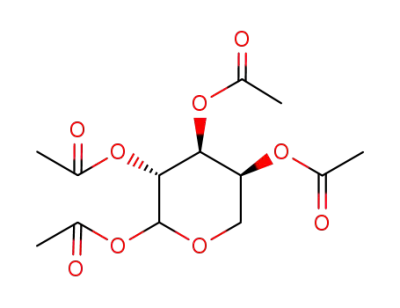
Reliable Quality α-D-Galactose Pentaacetate 4163-59-1 Hot Sale with Chinese Manufacturer
Synonyms:α-D-Galactose pentaacetate;Pentaacetyl-α-D-galactopyranose,;Pentaacetyl α-D-galactose;1,2,3,4,6-Penta-O-acetyl-α-D-galactopyranose
Molecular Formula: C16H22O11
Molecular Weight: 390.34
CAS No.: 4163-59-1
EC No.:609-944-3
Specification:
|
Item
|
Specification
|
|
Appearance
|
White crystalline powder
|
|
Assay(GC),%
|
≥98.0
|
|
Melting Point, ℃
|
92.0~98.0
|
|
Specific rotation,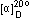 (C=1,CHCl3) (C=1,CHCl3)
|
+103.0~+107.0
|
|
Water,%
|
≤1.0
|
|
Residue on ignition, %
|
≤0.1
|
1,2,3,4,6-Penta-o-acetyl-alpha-d-galactopyranose(Cas 4163-59-1) Usage
α-D-Galactose pentaacetate is mainly used as medicine intermediate. As follows:
1. It is used for the preparation of galactose derivative cationic liposome nanoparticles to meet basic requirements of nucleic acid drug transporters.
2. It is used for recyclable binders for metal casting molds and for injection molding of metal and ceramic parts.
4163-59-1 Relevant articles
Selectivity of 1-O-Propargyl-D-Mannose Preparations
?ezanka, Michal,Dolensky, Bohumil,Krabicová, Ilona
, (2022/03/01)
Thanks to their ability to bind to speci...
Design, Synthesis, biological investigations and molecular interactions of triazole linked tacrine glycoconjugates as Acetylcholinesterase inhibitors with reduced hepatotoxicity
Ahmed, Ajaz,Bhagat, Kavita,Choudhary, Sushil,Kaur Gulati, Harmandeep,Kumar, Ajay,Kumar, Nitish,Mukherjee, Debaraj,Singh Bedi, Preet Mohinder,Singh, Atamjit,Singh, Harbinder,Vir Singh, Jatinder
, (2021/11/23)
Tacrine is a known Acetylcholinesterase ...
Synthesis and biological evaluation of 3β-O-neoglycosides of caudatin and its analogues as potential anticancer agents
Li, Xiao-San,Chen, Tang-Ji,Xu, Zhi-Peng,Long, Juan,He, Miao-Ying,Zhan, He-Hui,Zhuang, Hai-Cai,Wang, Qi-Lin,Liu, Li,Yang, Xue-Mei,Tang, Jin-Shan
, (2021/12/30)
In order to study the structure–activity...
PROCESS OF SYNTHESIS OF β-6'SULFOQUINOVOSYL DIACYLGLYCEROLS
-
Page/Page column 11; 12, (2022/02/28)
The present invention relates to a synth...
4163-59-1 Process route
-
- 2478-38-8
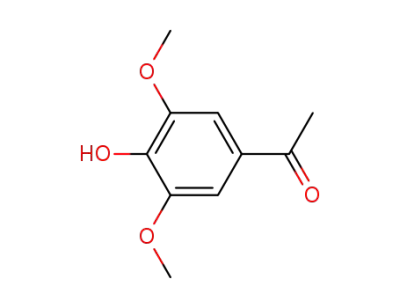
1-(4-hydroxy-3,5-dimethoxy-phenyl)-ethanone
-
- 108-24-7
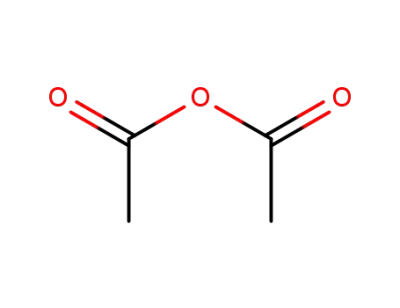
acetic anhydride
-
- 572-09-8
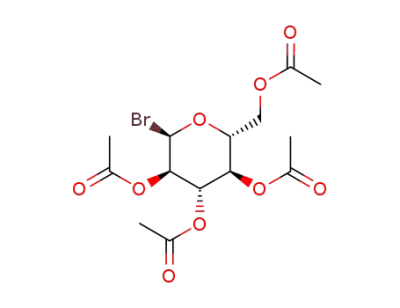
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide
-
- 83-87-4,604-68-2,604-69-3,2152-77-4,4026-35-1,4163-59-1,4163-60-4,4163-61-5,4163-65-9,4257-94-7,4257-96-9,16299-15-3,19186-39-1,19189-55-0,25878-60-8,25941-03-1,32445-48-0,32445-49-1,32445-53-7,32445-54-8,34685-58-0,34685-59-1,43168-42-9,43168-44-1,43169-22-8,43169-25-1,66966-07-2,70749-17-6,80184-01-6,93221-01-3,93221-02-4,99630-88-3,109215-53-4,115792-70-6,115792-73-9,139894-92-1,139973-25-4,144071-49-8,147648-81-5
D-glucose pentaacetate
-
- 28294-47-5
1-(4-acetoxy-3,5-dimethoxy-phenyl)-ethanone
-
- 86402-42-8
3,5-dimethoxy-4-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyloxy)acetophenone
Conditions
| Conditions |
Yield |
|
Yield given. Multistep reaction;
|
|
-
- 108-24-7
acetic anhydride
-
- 83-87-4,604-68-2,604-69-3,2152-77-4,4026-35-1,4163-59-1,4163-60-4,4163-61-5,4163-65-9,4257-94-7,4257-96-9,16299-15-3,19186-39-1,19189-55-0,25878-60-8,25941-03-1,32445-48-0,32445-49-1,32445-53-7,32445-54-8,34685-58-0,34685-59-1,43168-42-9,43168-44-1,43169-22-8,43169-25-1,66966-07-2,70749-17-6,80184-01-6,93221-01-3,93221-02-4,99630-88-3,109215-53-4,115792-70-6,115792-73-9,139894-92-1,139973-25-4,144071-49-8,147648-81-5
D-glucose pentaacetate
-
- 1233-03-0,2595-11-1,4026-34-0,4049-33-6,4049-34-7,4257-95-8,4257-98-1,4258-00-8,4627-30-9,17080-99-8,19186-37-9,25227-11-6,25243-38-3,62446-93-9,62929-49-1,67226-03-3,78087-60-2,78088-17-2,82890-16-2,86782-34-5,86782-35-6,92218-63-8,99880-95-2,108646-05-5,115939-79-2,142130-89-0,123163-97-3
1,2,3,4-tetra-O-acetyl-L-arabinopyranose
Conditions
| Conditions |
Yield |
|
nudicauloside B; With hydrogenchloride; water; for 4h; Reflux;
With methyldioctylamine; In chloroform; water;
acetic anhydride; With pyridine; for 24h;
|
|
4163-59-1 Upstream products
-
7322-31-8
β-D-mannose
-
108-24-7
acetic anhydride
-
3458-28-4
D-Mannose
-
530-26-7
D-Mannose
4163-59-1 Downstream products
-
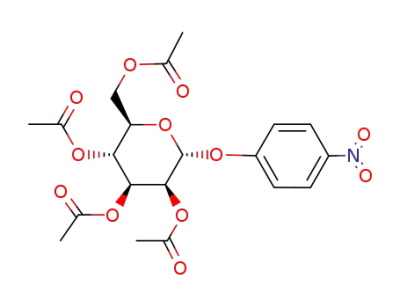
13242-51-8
1-O-p-nitrophenyl-2,3,4,6-tetra-O-acetyl α-D-mannopyranoside
-
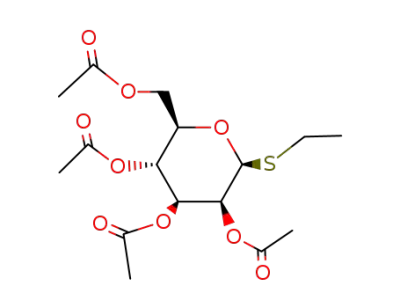
125354-48-5
ethyl 2,3,4,6-tetra-O-acetyl-1-thio-β-D-mannopyranoside
-
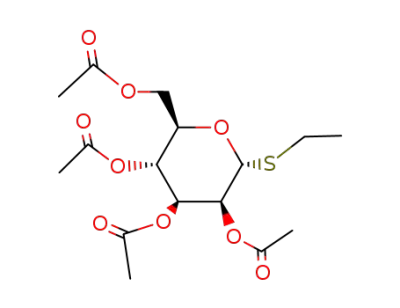
32934-24-0
S-ethyl 2,3,4,6-tetra-O-acetyl-1-deoxy-1-thio-α-D-mannopyranoside
-
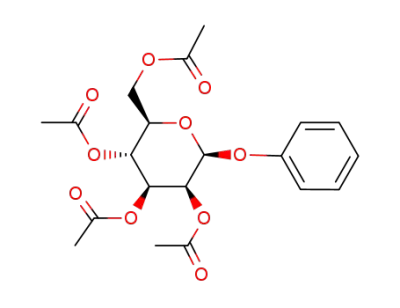
37797-55-0
1-O-phenyl-2,3,4,6-tetra-O-acetyl-β-D-mannopyranoside