Your Location:Home > Products > Medical intermediate > β-D-Galactose Pentaacetate
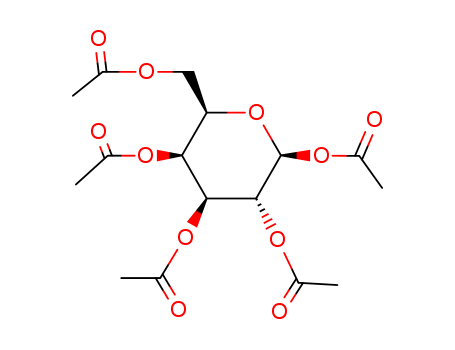

CasNo: 4163-60-4
MF: C16H22O11
Appearance: white fine crystalline powder
Packing: 25kgs/drum or as required by the customer.
Purity: ≥98
Synonyms:β-D-Galactose pentaacetate;Pentaacetyl-β-D- galactopyranose;Pentaacetyl β-D- galactose;1,2,3,4,6-Penta-O-acetyl-β-D- galactopyranose
Molecular Formular: C16H22O11
Molecular weight: 390.34
CAS No.: 4163-60-4
EC No.: 224-008-0
Molecular Structure:
Quality Standard:
|
Item |
Specification |
|
Appearance |
White crystalline powder |
|
Assay(GC),% |
≥98 |
|
Specific rotation, |
+23~+26 |
|
Melting Point, ℃ |
142~146 |
|
Water,% |
≤1.0 |
|
Residue on ignition, % |
≤0.1 |
|
Heavy metals,ppm |
≤10 |
Properties: It is White crystalline powder, it is insoluble in water and easy to soluble in ethanol, chloroform etc.
β-D-Galactose pentaacetate is mainly used in biochemical reaction and used as medicine intermediate. As follows:
1. β-D-Galactose pentaacetate used in the synthesis of 3-carbethoxyphenyl-beta-D-acetylated galactoside. The compound is used for depression treatment.
2. It used in the synthesis of 1,2-glycoside transderivative of oxazole compounds. It can be applied as antineoplastic medicine inhibiting microtubule assembly and selectively targeting tumor blood vessels.
3. It used in the synthesis of a rupestonic acid glycolipid derivative. The derivative is prepared by synthetizing rupestonic acid and fully acetylized glycosyl bromide as raw materials. The test result shows that compounds 2d and 2g have better inhibitory action on the influenza A (H3N2) viruses, and by-products 3 have better inhibitory action on influenza A (H3N2 and H1N1) and B viruses.
4. It used in the synthesis of β-D-galactosamine pentaacetate as medicine intermediate.
5. Synthesis of galactose modified nitric oxide slow-release hydrogel for the treatment of periodontitis
6. Synthesis of novel trimethylcitryl--D-galactopyranoside has inhibitory effects on central nervous system.
7. The zein and glycopolypeptide grafted copolymer prepared by means of the preparation method can be used for preparing a corresponding electrospun fibrous membrane serving as a liver cell scaffold material by adopting an electrospinning technique.
8. Synthesis of 18F-labeled galactose derivative as a novel PET probe for imaging asialoglycoprotein receptor, where the probe can be an alternative glucose metabolism imaging agent used in clinic examination and quantification.
9. Synthesis of novel sulfated sugar polymers for the treatment and/or regulation of inflammation, or for the treatment and/or prevention of ischemia, including the treatment and/or prevention of ischemia-reperfusion injury or ischemic stroke, the treatment of cancer, and for organ transplantation.
10.Used for the synthesis of tenofovir derivatives, has antiviral effect, can concentrate in the liver and slowly release the active ingredients, drug action is highly effective, low toxicity.
11. The structure and drug delivery system of a nano-micelle with double ligand reversible shielding were synthesized for the treatment of cancer.
12. It can be used as the material of proximal cap of vascular closure plug.
13. It used in the synthesis of isopropyl-beta-D-thiogalactoside (IPTG) as a powerful inducer. Or synthesis of more stable inducible protein expression of thiogalactoside IBCG, used to induce gene expression to produce a recombinant protein as a functional substitute for IPTG.
14. It used in the synthesis of 4-methyl umbrella-shaped keto-beta-D-pyran galactoside. It’s an important fluorescent substrate used to detect coliforms in water and food. As the glycosidase substrate, it can be used to analyze the activity of the corresponding glycosidase and to study the microbiological detection of the corresponding specific glycosidase as an important indicator.
15. A β-galactosidase coloring substrate prepared by using 4-nitro-1-naphthol or a derivative thereof as a chromophore.
16. It is used for the preparation of fluorescent compounds, and use the fluorescent compound to prepare a sensing film to realize sensitive detection of hydrogen chloride vapor.
17. It used in the synthesis of 1-thio-phenyl-2,3,4-tri-O-benzyl-O-substituent acyl-1-deoxy-β-D-galactose ester as the galactose ester-based donor.
18. It is used for the preparation of polysaccharides connected by the novel oxime and the preparation of 6-azide -2, 4-diacetyl amino-2, 4, 6-trideoxy d-mannose.
19. Preparation of glycosylated nanofibers for protein recognition.
20. It is used for recyclable binders for metal casting molds and for injection molding of metal and ceramic parts.
Storage: St
ore in a tightly closed container.Store in a cool,dry area.
Package: 25kgs/drum or as required by the customer.
Expiration Date: 2 years.
Enzyme modifiable, hollow self-assembled...
The gram-negative bacterial pathogen Pse...
The development of a galactose-capped go...
Glycosidases are key enzymes in metaboli...
Epothilone, the macrolide compound produ...
4,6-O-Benzylidene-α-d-galactosyl azide c...
Tacrine is a known Acetylcholinesterase ...
Thanks to their ability to bind to speci...
In order to study the structure–activity...
The present invention relates to a synth...
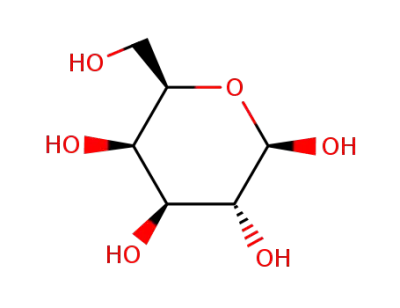
β-D-galactopyranoside

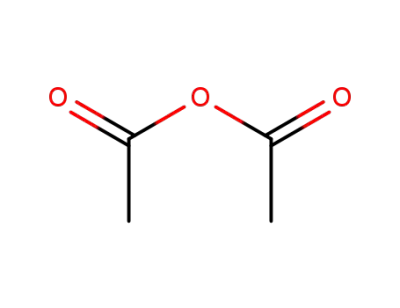
acetic anhydride

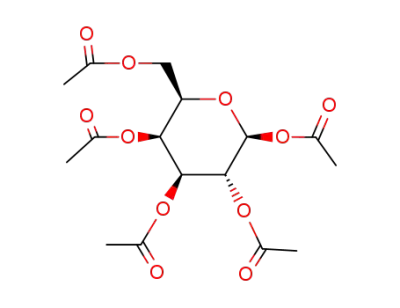
β-D-galactose peracetate
| Conditions | Yield |
|---|---|
|
With perchloric acid; In dichloromethane; at 0 ℃; for 1h;
|
100% |
|
With sodium acetate; at 120 ℃; for 1h;
|
96% |
|
acetic anhydride; With sodium acetate; at 100 ℃; for 0.5h;
β-D-galactopyranoside; at 100 ℃; for 1h;
|
96% |
|
With pyridine; at 20 ℃; for 24h;
|
95% |
|
With tris(pentafluorophenyl)borate; In neat (no solvent); at 20 ℃; for 0.333333h; Green chemistry;
|
92% |
|
With sodium acetate; for 1.5h; Reflux;
|
85% |
|
With sodium acetate; at 90 ℃; for 4h;
|
77% |
|
With pyridine; at 20 ℃; Inert atmosphere;
|
65.8% |
|
With sodium acetate; Reflux;
|
47% |
|
With pyridine; at 4 - 20 ℃;
|
45% |
|
|
|
|
With iodine; at 20 ℃;
|
|
|
With sodium acetate; at 60 ℃; for 12h;
|
|
|
With pyridine; at 20 ℃; for 24h;
|
|
|
With sodium acetate; at 129 ℃; for 2.5h;
|
|
|
With sodium acetate;
|
|
|
With sodium acetate;
|
|
|
With pyridine;
|
|
|
With pyridine;
|
|
|
With sodium acetate; Reflux;
|
|
|
With iodine;
|
|
|
With iodine; at 25 ℃;
|
|
|
at 25 ℃;
|
|
|
With pyridine; triethylamine; for 4h;
|
|
|
With iodine; at 20 ℃;
|
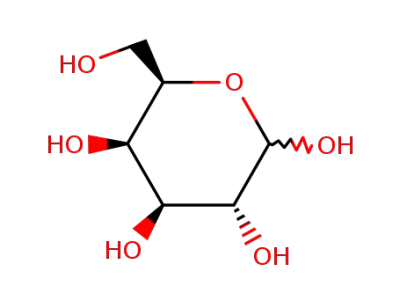
D-Galactose


acetic anhydride


β-D-galactose peracetate
| Conditions | Yield |
|---|---|
|
sulfuric acid; at 20 ℃; for 0.0833333h; sonication;
|
94% |
|
With sodium acetate; at 120 ℃;
|
94% |
|
With sodium acetate; for 0.00555556h; microwave irradiation;
|
92% |
|
With sodium acetate; at 90 ℃; for 4h;
|
92% |
|
With sodium acetate; at 90 ℃; for 4h;
|
92% |
|
With sodium acetate; at 60 ℃; for 12h;
|
90% |
|
With sodium acetate; at 128 ℃;
|
90% |
|
With sodium acetate; at 140 ℃; for 1h;
|
88% |
|
With sodium acetate; for 4h; Reflux;
|
83% |
|
With sodium acetate; at 60 ℃; for 12h;
|
82% |
|
With sodium acetate; at 60 ℃;
|
82% |
|
With sodium acetate; for 1h; Reflux;
|
81% |
|
With pyridine; dmap; at 20 ℃;
|
75% |
|
With sodium acetate; Reflux;
|
47% |
|
acetic anhydride; With sodium acetate; at 120 ℃; for 0.5h;
D-Galactose; for 2h;
|
31% |
|
With sodium acetate; at 100 ℃; for 4h;
|
|
|
With iodine;
|
|
|
With sodium acetate; Reflux;
|
|
|
With tin(IV) chloride; at 20 ℃; for 0.166667h;
|
|
|
With sodium acetate; Reflux;
|
|
|
With sodium acetate; for 5h; Reflux;
|
|
|
Alkaline conditions;
|
|
|
With pyridine; dmap; at 0 - 20 ℃; for 13h;
|
|
|
With sodium acetate; at 100 ℃;
|
|
|
With sodium acetate;
|
|
|
With sodium acetate; for 1h;
|
|
|
With iodine; for 8h;
|
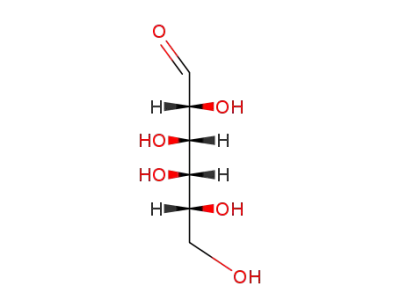
D-Galactose

acetic anhydride

diazomethane

D-Galactose
1-O-p-nitrophenyl-2,3,4,6-tetra-O-acetyl α-D-mannopyranoside
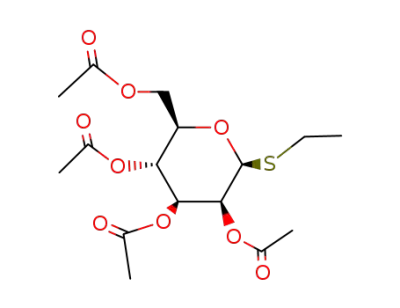
ethyl 2,3,4,6-tetra-O-acetyl-1-thio-β-D-mannopyranoside
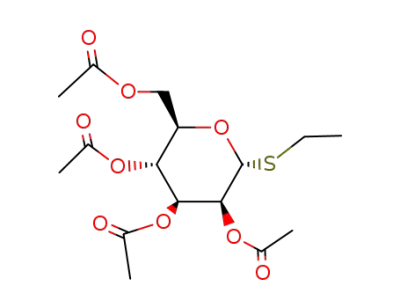
S-ethyl 2,3,4,6-tetra-O-acetyl-1-deoxy-1-thio-α-D-mannopyranoside
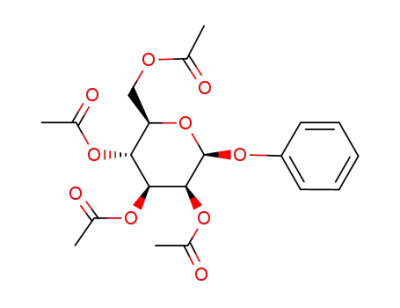
1-O-phenyl-2,3,4,6-tetra-O-acetyl-β-D-mannopyranoside