Your Location:Home > Products > Medical intermediate > Galactose Pentaacetate
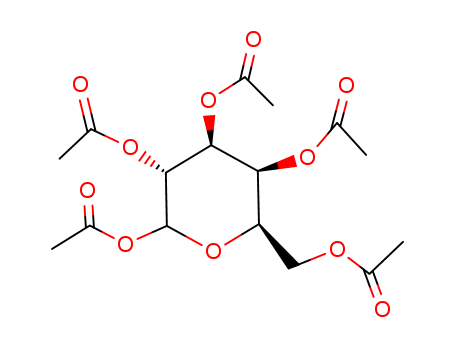

CasNo: 25878-60-8
MF: C16H22O11
Packing: 25kg/drum or as required by customer
Molecular Formula:C16H22O11
Molecular Weight:390.34
CAS Number:25878-60-8
|
Item |
Specification |
|
Appearance |
White crystalline powder |
|
Assay (GC),% |
≥98.0 |
|
Loss on drying,% |
≤1.0 |
|
Residue on ignition,% |
≤0.10 |
Characteristics: It is white crystalline powder. It is easily soluble in ethanol, chloroform and insoluble in water.
α,β-D-Galactose pentaacetate is mainly used as medicine intermediate. As follows:
α,β-D-Galactose pentaacetate is an analog of the natural pentoses that binds to the mitochondrial membrane and inhibits the production of pro-inflammatory cytokines. This drug has been shown to inhibit the binding of lysophosphatidic acid (LPA) to its receptor by substituting for LPA in this binding site. α,β-D-Galactose pentaacetate also inhibits the expression of proinflammatory cytokines such as interleukin 6 (IL6) and IL1β in a dose dependent manner. This drug is also capable of inhibiting phosphotungstic acid from binding to a monolayer surface and can be used as a glycopolymer for cell culture.
Storage: Store in a tightly closed container. Maintain in a cool and dry area.
Package: 25kg/drum or as required by customer.
Expiration Date: 2 years
Thanks to their ability to bind to speci...
Tacrine is a known Acetylcholinesterase ...
In order to study the structure–activity...
The present invention relates to a synth...
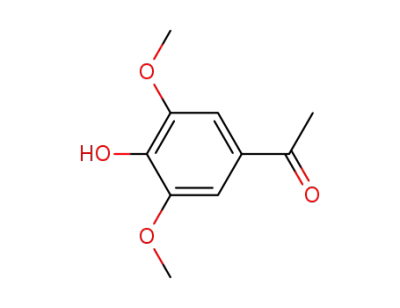
1-(4-hydroxy-3,5-dimethoxy-phenyl)-ethanone

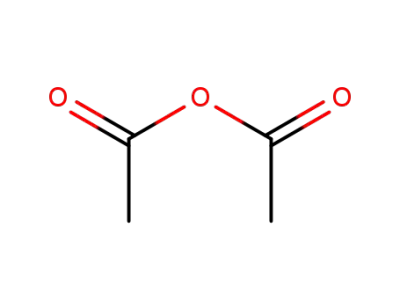
acetic anhydride

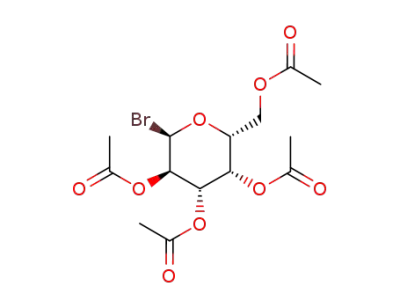
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

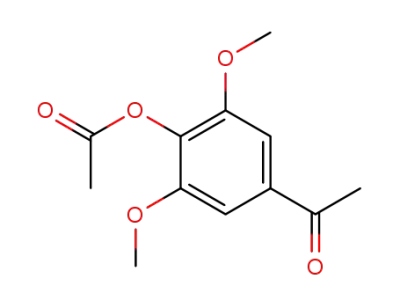
1-(4-acetoxy-3,5-dimethoxy-phenyl)-ethanone

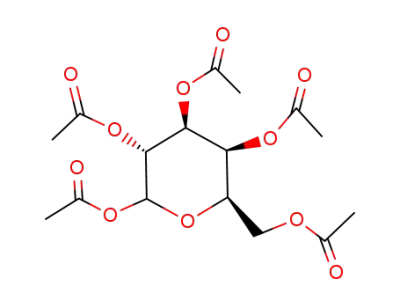
D-galactose pentaacetate

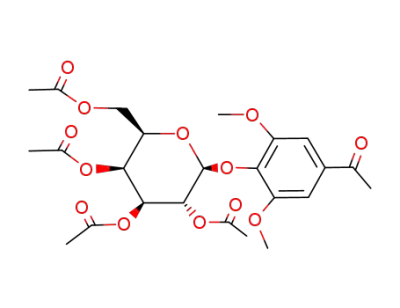
3,5-dimethoxy-4-(2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyloxy)acetophenone
| Conditions | Yield |
|---|---|
|
Yield given. Multistep reaction;
|

1-(4-hydroxy-3,5-dimethoxy-phenyl)-ethanone


acetic anhydride

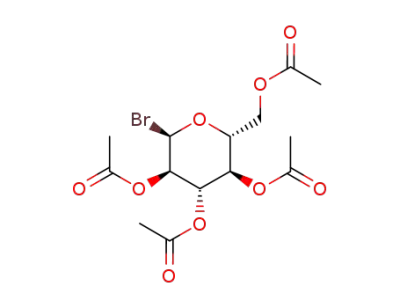
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

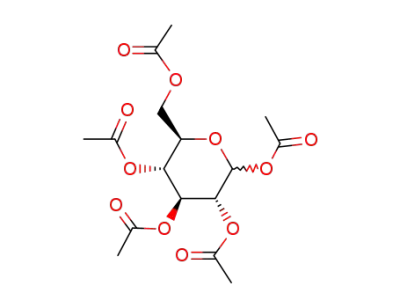
D-glucose pentaacetate


1-(4-acetoxy-3,5-dimethoxy-phenyl)-ethanone

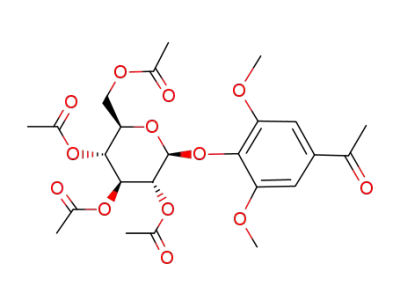
3,5-dimethoxy-4-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyloxy)acetophenone
| Conditions | Yield |
|---|---|
|
Yield given. Multistep reaction;
|
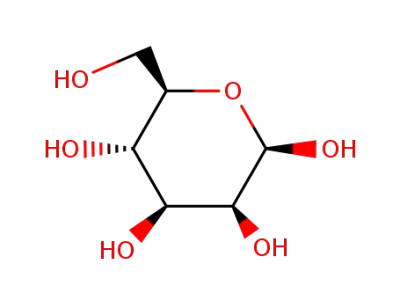
β-D-mannose

acetic anhydride
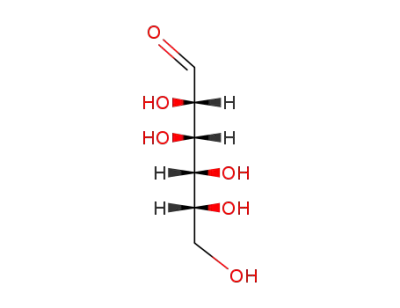
D-Mannose
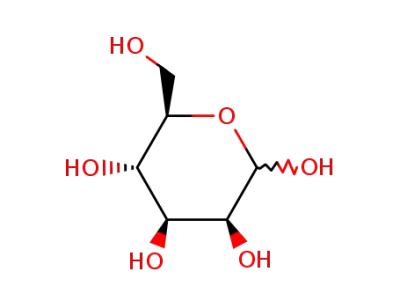
D-Mannose
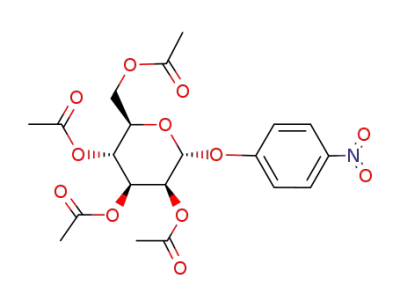
1-O-p-nitrophenyl-2,3,4,6-tetra-O-acetyl α-D-mannopyranoside
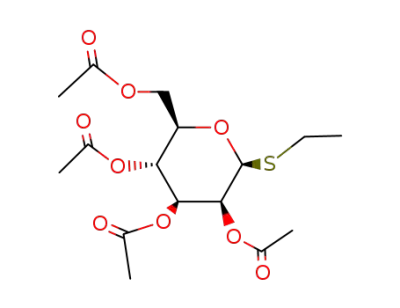
ethyl 2,3,4,6-tetra-O-acetyl-1-thio-β-D-mannopyranoside
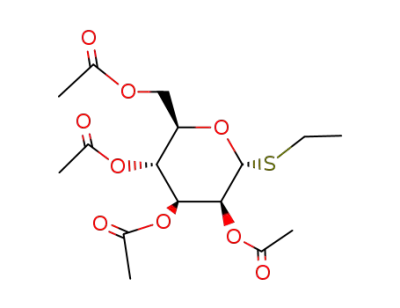
S-ethyl 2,3,4,6-tetra-O-acetyl-1-deoxy-1-thio-α-D-mannopyranoside
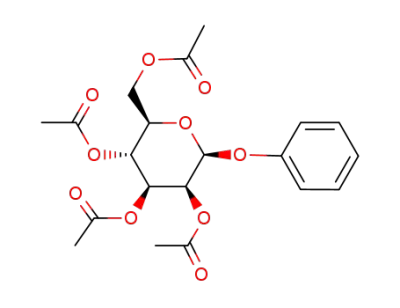
1-O-phenyl-2,3,4,6-tetra-O-acetyl-β-D-mannopyranoside