Your Location:Home > Products > Medical intermediate > Diacetone-D-Galactose

CasNo: 4064-06-6
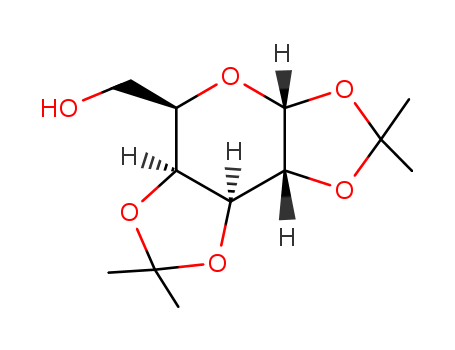
MF: C12H20O6
Appearance: Thick yellow oil
Packing: 25kg/drum or as required by customer.
Purity: 99%
Synonym: 1,2:3,4-Di-O-isopropylidene-α-D-galactopyranose; 1,2:3,4-Di-O-isopropylidene-D-galactopyranose;1,2:3,4-Di-O-isopropylidene-α-D-galactose; 1,2:3,4-Di-O-isopropylidene-D-galactose;1,2:3,4-O-Diisopropylidene-α-D-galactopyranose; 1,2:3,4-bis-O-(1-methylethylidene)-D-galactopyranos; Diisopropylidenegalactose.
Molecular Formula: C12H20O6
Molecular weight: 260.28
CAS No.: 4064-06-6
EC No.: 223-771-7
Molecular structure:
Quality Standard:
|
Item |
Specification |
|
Appearance |
Colorless to yellow viscous liquid |
|
Assay (GC), % |
≥95.0 |
|
Specific rotation , |
-55.0~-61.0 |
|
Loss on drying, % |
≤1.0 |
|
Residue on ignition, % |
≤0.1 |
Properties: Colorless to yellow viscous liquid. Dissolve in hot water, acetone, ethanol, tetrahydrofuran etc.
As a derivative of natural sugar ,it has been widely used in medicine ,materials, dyes, spices and so on.
Can be used as pharmaceutical intermediates for the synthesis of carbohydrate drugs which are usually low toxic and side effect and high biological activity. used for the treatment of skin diseases and used in cosmetic provided with anti-wrinkle,anti-aging,smooth etc,and dermatopathya treatment. Modification of hydrocodone, opium and other analgesic drugs, can enhance the efficacy or reduce addiction, to reduce the control of drug abuse objective.
It also be used for the synthesis of biological probes which have wide application prospect in pharmaceutical and medical aspects. Such as modification of porphyrin and the formation of the carbohydrate porphyrin compounds, has excellent photophysical behavior and biocompatibility, which can be used for near infrared probe and a photosensitive drug, diagnosis and treatment of tumors.
Diacetone galactose can also be used in the synthesis of functional polymer materials. Also can be used for the synthesis of a new environmentally friendly dye, Used in synthesis of perfluoroalkylated derivatives of D-galactopyranose. Used in synthesis terpenoid-O-glycosides, which are food spice and Perfume.
Storage: Store in a tightly closed container. Store in a cool and dry area.
Package: 25kg/drum or as required by customer.
-
-
A remarkably efficient synthesis of the ...
Biofilm formation by pathogenic bacteria...
-
A simple and mild system using 4,4′-bis-...
A galactose containing glycomonomer has ...
The synthesis of 5-[6'-deoxy-(1',2':3',4...
-
Mono- and diacylated derivatives of gala...
The title organotin carbohydrate, C31H36...
Four galactoconjugated zinc(II) phthaloc...
-
The solution-state conformations of vari...
A Me3SI-mediated simple and efficient pr...
This study presents the novel concept of...
A phenylselenoglycosylation reaction of ...
Bioorthogonal decaging reactions for con...
![(3aR,5R,5aS,8aS,8bR)-5-(Bromo-phenyl-methoxymethyl)-2,2,7,7-tetramethyl-tetrahydro-bis[1,3]dioxolo[4,5-b;4',5'-d]pyran](/upload/2026/1/b0d8b6b6-5c63-48f9-85f7-a346659ce674.png)
(3aR,5R,5aS,8aS,8bR)-5-(Bromo-phenyl-methoxymethyl)-2,2,7,7-tetramethyl-tetrahydro-bis[1,3]dioxolo[4,5-b;4',5'-d]pyran

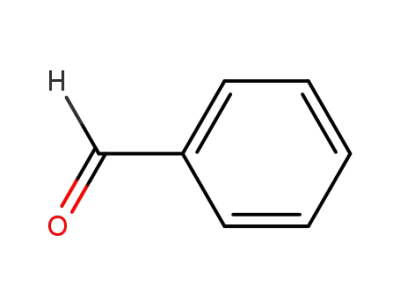
benzaldehyde

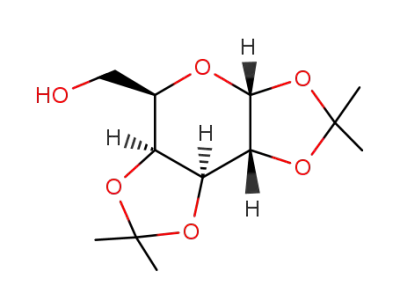
1,2:3,4-di-O-isopropylidene-α-D-galactopyranose
| Conditions | Yield |
|---|---|
|
With water; Yield given;
|
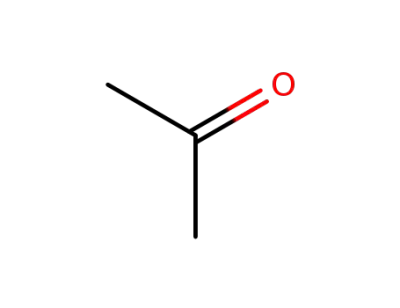
acetone

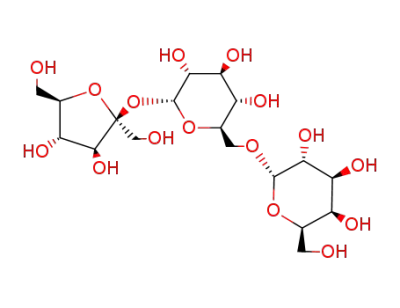
D-raffinose

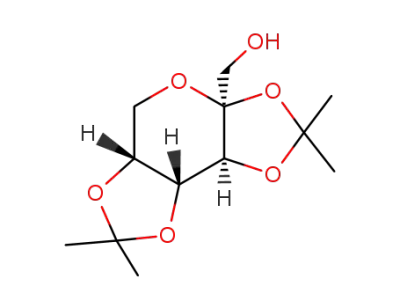
2,3;4,5-di-O-isopropylidene-β-D-fructopyranose

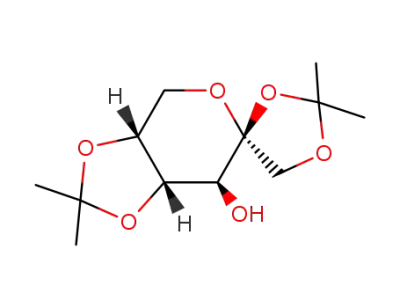
1,2;4,5-di-O-isopropylidene-β-D-(-)-fructopyranose


1,2:3,4-di-O-isopropylidene-α-D-galactopyranose

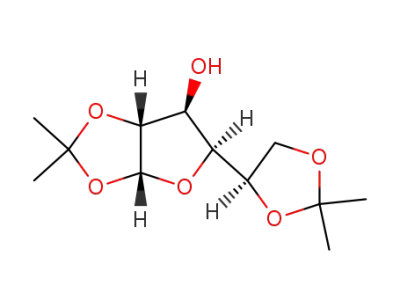
1,2:5,6-di-O-isopropylidene-α-D-glucofuranose
| Conditions | Yield |
|---|---|
|
iodine; for 72h; Title compound not separated from byproducts; Heating;
|
27% 37% 28% 4 % Chromat. |
|
iodine; for 72h; Title compound not separated from byproducts; Heating;
|
27% 4% 28% 37 % Chromat. |
|
iodine; for 72h; Title compound not separated from byproducts; Heating;
|
27 % Chromat. 37 % Chromat. 4 % Chromat. 28 % Chromat. |
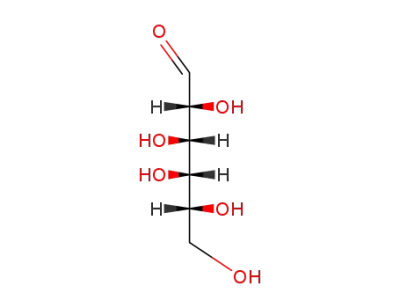
D-Galactose
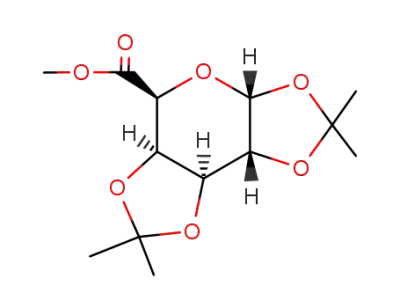
(3aR,5S,5aR,8aS,8bR)-methyl-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4',5'-d]pyran-5-carboxylate
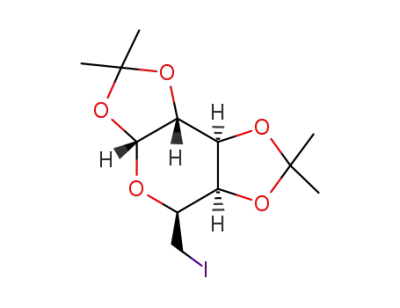
1,2:3,4-di-O-isopropylidene-6-iodo-D-galactopyranose
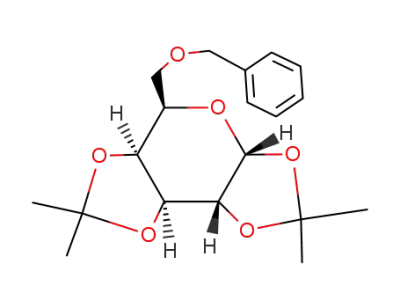
6-O-benzyl-1,2:3,4-di-O-isopropylidene-α-D-galactopyranose
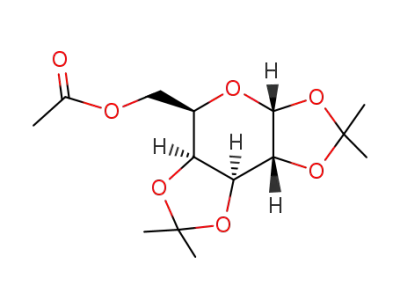
6-O-acetyl-1,2:3,4-di-O-isopropylidene-α-D-galactopyranose
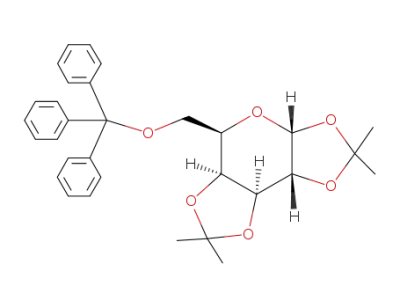
1,2:3,4-di-O-isopropylidene-6-O-trityl-α-D-galactopyranose
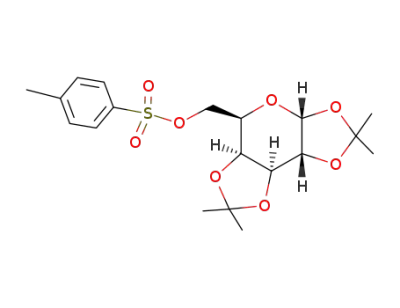
(1,2:3,4-di-O-isopropylidene-6-O-(p-toluensulfonate))-D-galactopiranose
diphenyl (1,2:3,4-di-O-isopropylidene-α-D-galactopyranosyl)-6-O-phosphate