Your Location:Home > Products > Medical intermediate > α-D-Mannose Pentaacetate
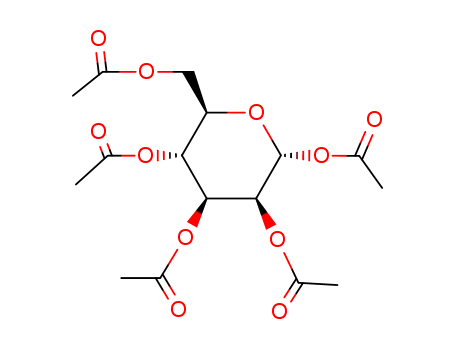

CasNo: 4163-65-9
MF: C16H22O11
Packing: customer require
Synonyms: Penta-O-acetyl-alpha-D-mannopyranose; 1,2,3,4,6-Penta-O-acetyl-alpha-D-mannose; alpha-D-Mannopyranose pentaacetate; D-alpha-Mannose pentaacetate; 1,2,3,4,6-penta-O-acetyl-alpha-D-mannopyranose; D-Mannose Pentacetate; α-D-mannose pentaacetate; 1,2,3,4,6-Penta-O-acetyl-D-Mannopyranose.
Molecular Formula: C16H22O11
Molecular Weight: 390.34
CAS Number: 4163-65-9
| Item | Specification |
|---|---|
| Appearance | White to off-white crystalline powder |
| Assay(GC), % | ≥98.0 |
| Melting point, °C | 64.0~75.0 |
Specific rotation , 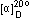
(C=1,CHCl3) |
+51.0~+57.0 |
| Water,% | ≤1.0 |
| Residue on ignition, % | ≤0.10 |
Characteristics: The product is a White to off-white crystalline powder. It is readily soluble in CHCl3 and insoluble in water.
This product can be used to synthesis the mannose-conjugated (trans-R,R-cyclohexane-1,2-diamine)-2-flouromalonato-platinum(II) complex which shows cytotoxicity to MCF-7 cell line, mannose-cationic conjugated polymers, photodynamic therapy agents such as amphiphilic glycosylated lipid porphyrin, photoluminescent functionalized silicon nanocrystals bearing D-mannose for cancer cells imaging, polymeric drug delivery systems that containing mannose-based, the anti-inflammatory, analgesic activity of tocopherol, morphine and targeting of bleomycin are both increased when they contain mannopyranoyl- or conjugate carbamoylmannose moiety. It is also used to synthesis thioglycoside, N-glycoside of mannose and bromo-tetra-O-acetyl mannopyranose and so on.
Package: customer require.
Expiration Date: 2 years
The present invention relates to a synth...
Thanks to their ability to bind to speci...
Tacrine is a known Acetylcholinesterase ...
In order to study the structure–activity...
1-(4-hydroxy-3,5-dimethoxy-phenyl)-ethanone

acetic anhydride

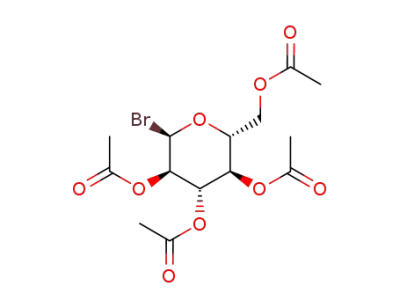
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

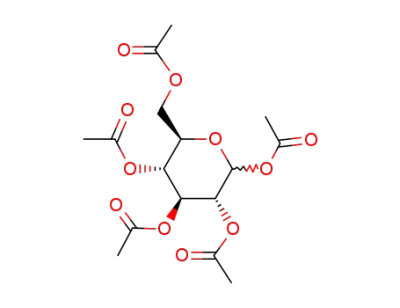
D-glucose pentaacetate

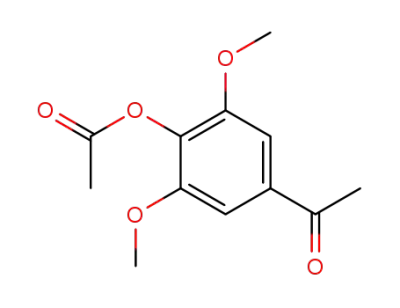
1-(4-acetoxy-3,5-dimethoxy-phenyl)-ethanone

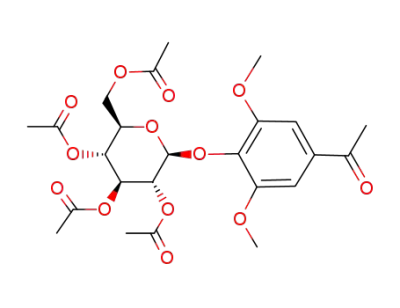
3,5-dimethoxy-4-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyloxy)acetophenone
| Conditions | Yield |
|---|---|
|
Yield given. Multistep reaction;
|
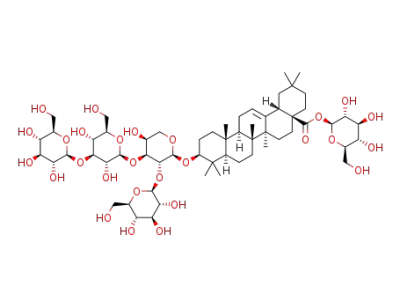
nudicauloside B


acetic anhydride


D-glucose pentaacetate

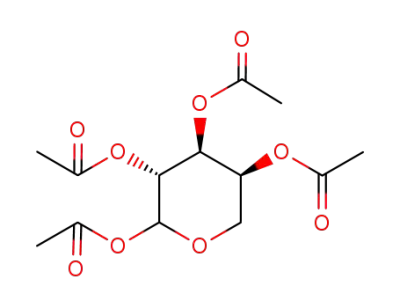
1,2,3,4-tetra-O-acetyl-L-arabinopyranose
| Conditions | Yield |
|---|---|
|
nudicauloside B; With hydrogenchloride; water; for 4h; Reflux;
With methyldioctylamine; In chloroform; water;
acetic anhydride; With pyridine; for 24h;
|
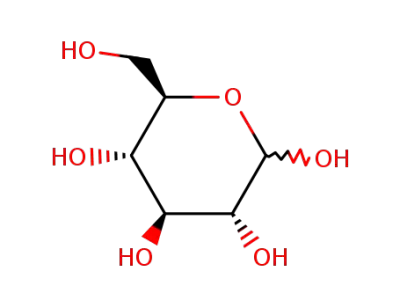
D-Glucose

acetic anhydride
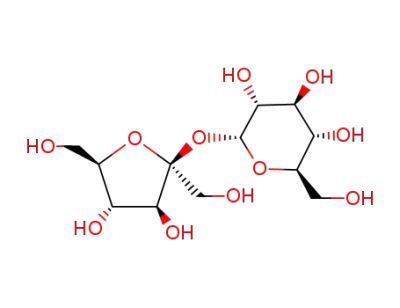
Sucrose

1-(4-hydroxy-3,5-dimethoxy-phenyl)-ethanone
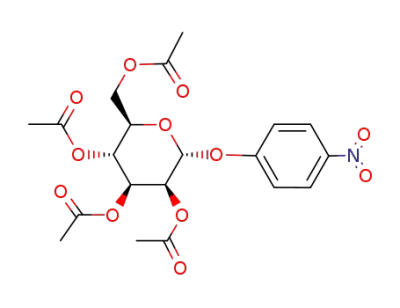
1-O-p-nitrophenyl-2,3,4,6-tetra-O-acetyl α-D-mannopyranoside
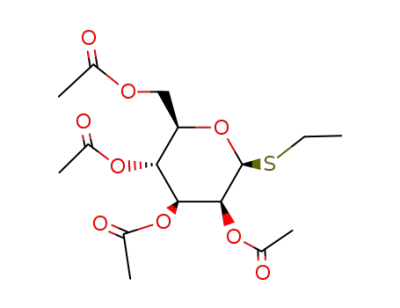
ethyl 2,3,4,6-tetra-O-acetyl-1-thio-β-D-mannopyranoside
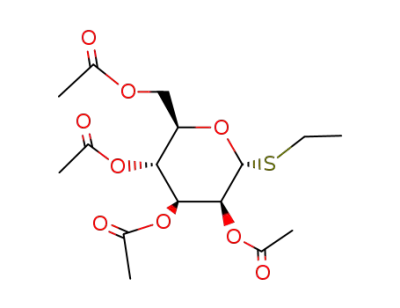
S-ethyl 2,3,4,6-tetra-O-acetyl-1-deoxy-1-thio-α-D-mannopyranoside
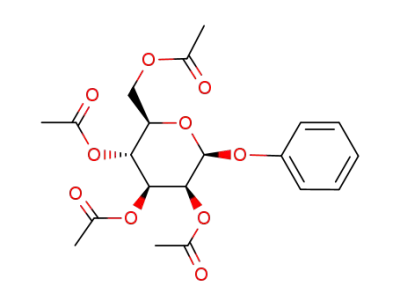
1-O-phenyl-2,3,4,6-tetra-O-acetyl-β-D-mannopyranoside