Your Location:Home > Products > Medical intermediate > 3-O-Benzyl- 1,2:5,6-Di-O-Isopropylidene-α-D-Glucofuranose
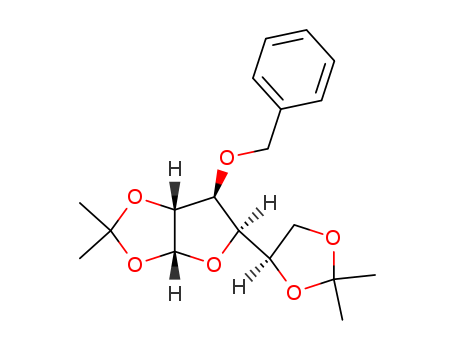

CasNo: 18685-18-2
MF: C19H26 O6
Appearance: Colourless syrup
Synonyms: 1,2:5,6-bis-O-(1-methylethylidene)-3-O-(phenylmethyl)- a-D-Glucofuranose; 1,2:5,6-di-O-isopropylidene-3-O-benzyl-α-D-Glucofuranose; 3-O-benzyl-1,2:5,6-di-O-isopropylidene-D-Glucofuranose; Furo[2,3-d]-1,3-dioxole, a-D-glucofuranose deriv.
Molecular Formula: C19H26O6
Molecular weight: 350.4061
CAS No.: 18685-18-2
Storage: Maintain in cool, dry containment.
Package: 25kg/drum or as customer require.
Expiration Date: 2 years
Quality Standard:
| Item | Specification |
|---|---|
| Appearance | Sticky liquid of yellow to wine red |
| Assay (GC), % | ≥98 |
Specific rotation 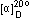 (C=1.0,CHCl3) (C=1.0,CHCl3) |
--24.5~-28.5 |
Characteristics: This product is a sticky liquid of yellow to wine red. It soluble in ethanol, chloroform, dichloromethane, ethyl acetate, and ethyl ether, etc., insoluble in water.
The product is an important materials and intermediates of pharmaceuticals. As start material which mainly used in synthesis anticancer agent Goniofufurone, brain-tumor inhibitor 2-deoxy-2,2-difluoro-D-arabino-hexopyranose, activated precursors of oligonucleotides (9-(3'-O-benzyl-2'-O-benzyloxycarbonyl)β-D-xylofuranosyl) adenine, glycosidase inhibitor nojirimycin, HIV inhibitor 1,4-Dideoxy-1,4-imino-D-arabinitol, anti-inflammatory agent such as glycosylated indole, (-) - Dinemasone B, phosphatidyl-myo-inostiol mannoside analogues, and glycosylated asterosaponin with mult-pharmacological activities, etc.. It used in synthesis following pharmaceutica intermediate 2,4,6-tri-O-benzoyl-3-O-benzyl glucopyranosyl imidate for glucan-hexasaccharide vaccines, 3-O-benzyl-1,2-O-isopropylidene-α-L-idofuranose for Fondaparinux sodium, and 3-O-Benzyl-1,2-O-isopropylidene-α-xylo-pentodialdofuranose for anthracyclinone. The product also used in synthesis its following derivatives 3-O-benzyl-1,2-O-isopropylidene-D-glucofuranose, 3-O-benzyl-D-glucofuranose, α,β-D-Methyl glucopyranoside, 2,5, 6-tri-O-methyl-D-glucofuranose, 3-O-benzyl-xylitol, 3,4-di-O-methyl-D-erythritol, 2,4,6-Tri-O-methyl-D-mannose, L-idose, 6-deoxy-6-Acetamido-L-idothiapyranose, 6-O-benzoyl-l,2-O-isopropylidene-D-glucofuranose,3-O-benzyl-4,6-benzylidene-D-glucofuranose, 3-O-benzyl-1,2-isopropylidene-α-D-glucopyranoic acid, Methyl-3-O-Benzyl-5,6-di-O-isopropyIidene-α,β-D-glucofuranoside and so on. In addition, the product also is a kind of chiral auxiliary for some organicreaction and synthon for oligosaccharides and glycopolymer
Synthesis of 2′-O,5′-C-bridged-β-D-homol...
-
(-)-Dinemasone B was isolated by Krohn a...
The synthetic route to 3-O-benzyl-6-O-pi...
Chiral monoaza-15-crown-5 lariat ethers ...
Using the “chiral pool” approach, two mo...
The cleavage of benzyl ethers by catalyt...
The invention belongs to the field of ch...
benzyl chloride

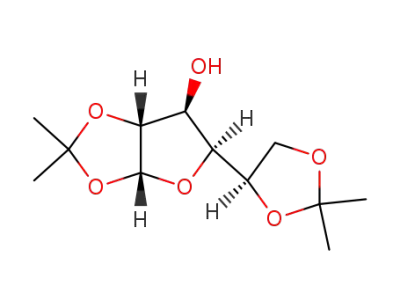
1,2:5,6-di-O-isopropylidene-α-D-glucofuranose

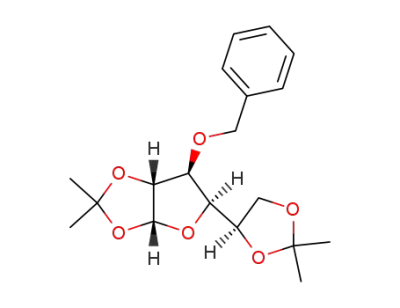
3-O-benzyl-1,2-5,6-O-diisopropylidene-α-D-glucofuranose
| Conditions | Yield |
|---|---|
|
With sodium hydride; In tetrahydrofuran; for 24h; Ambient temperature;
|
98% |
|
With potassium hydroxide; In acetonitrile; at 150 ℃; for 0.0333333h; microwave irradiation;
|
98% |
|
With sodium hydride; In water; N,N-dimethyl-formamide; mineral oil; at 20 ℃; for 0.5h;
|
97% |
|
With sodium hydroxide; tetrabutyl-ammonium chloride; In benzene; at 80 ℃; for 2h;
|
96% |
|
With sodium hydride; In tetrahydrofuran; dimethyl sulfoxide; for 3h; Heating;
|
95% |
|
1,2:5,6-di-O-isopropylidene-α-D-glucofuranose; With sodium hydride; In N,N-dimethyl-formamide; benzene; at 20 ℃; for 2h;
benzyl chloride; In N,N-dimethyl-formamide; benzene; at 20 ℃; for 16h; Further stages.;
|
71% |
|
With sodium hydride; In dimethyl sulfoxide;
|
|
|
With sodium hydride; Multistep reaction; 1.) THF, DMSO, RT, 15 min, 2.) THF, DMSO, RT, 35 min;
|
|
|
1,2:5,6-di-O-isopropylidene-α-D-glucofuranose; With sodium hydride; In N,N-dimethyl-formamide; for 0.5h;
benzyl chloride; In N,N-dimethyl-formamide; at 20 ℃; for 2h; Further stages.;
|
|
|
With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate; In tetrahydrofuran; water; for 3h; Heating / reflux;
|
|
|
With tetra(n-butyl)ammonium hydrogensulfate; sodium hydroxide; In tetrahydrofuran; for 2h; Reflux;
|
|
|
With tetrabutylammomium bromide; potassium hydroxide; In toluene; at 80 - 85 ℃;
|
149 g |
benzyl bromide


1,2:5,6-di-O-isopropylidene-α-D-glucofuranose


3-O-benzyl-1,2-5,6-O-diisopropylidene-α-D-glucofuranose
| Conditions | Yield |
|---|---|
|
With tetra-(n-butyl)ammonium iodide; sodium hydride; In tetrahydrofuran; at 20 ℃; for 5h;
|
100% |
|
1,2:5,6-di-O-isopropylidene-α-D-glucofuranose; With sodium hydride; In tetrahydrofuran; at 0 ℃; for 0.5h;
benzyl bromide; In tetrahydrofuran; at 40 ℃; for 10h;
|
100% |
|
With sodium hydride; In N,N-dimethyl-formamide; at 20 ℃; for 8h;
|
100% |
|
With sodium hydride; In N,N-dimethyl-formamide; at 0 - 24 ℃; for 2h;
|
100% |
|
With sodium hydride; In N,N-dimethyl-formamide; at 25 ℃; for 6h;
|
100% |
|
1,2:5,6-di-O-isopropylidene-α-D-glucofuranose; With sodium hydride; In N,N-dimethyl-formamide; mineral oil; at 0 - 20 ℃; Inert atmosphere;
benzyl bromide; In N,N-dimethyl-formamide; mineral oil; at 20 ℃; for 18h; Inert atmosphere;
|
99% |
|
With sodium hydride;
|
98% |
|
With sodium hydride; In tetrahydrofuran; at 50 ℃;
|
98% |
|
With sodium hydride; In N,N-dimethyl-formamide; at 0 - 20 ℃; for 2h;
|
98% |
|
With sodium hydride; In N,N-dimethyl-formamide; at 20 ℃; for 2.5h;
|
97% |
|
With sodium hydride; In acetonitrile; at 180 ℃; for 0.0166667h; microwave irradiation;
|
96% |
|
With barium dihydroxide; barium(II) oxide; In dimethyl sulfoxide; for 16h;
|
95% |
|
1,2:5,6-di-O-isopropylidene-α-D-glucofuranose; With potassium hydroxide; In dimethyl sulfoxide; for 0.5h;
benzyl bromide; In dimethyl sulfoxide; at 20 ℃; for 14h;
|
95% |
|
With sodium hydride; In tetrahydrofuran; mineral oil; at 0 - 20 ℃; for 2h;
|
95% |
|
1,2:5,6-di-O-isopropylidene-α-D-glucofuranose; With sodium hydride; In N,N-dimethyl-formamide; at 0 ℃; for 0.25h;
benzyl bromide; In N,N-dimethyl-formamide; at 0 ℃; for 0.5h;
|
94% |
|
1,2:5,6-di-O-isopropylidene-α-D-glucofuranose; With sodium hydride; In N,N-dimethyl-formamide; mineral oil; at 0 - 20 ℃; for 0.5h; Inert atmosphere;
benzyl bromide; In N,N-dimethyl-formamide; mineral oil; at 0 - 20 ℃; for 23h; Inert atmosphere;
|
94% |
|
With tetra-(n-butyl)ammonium iodide; sodium hydride; In tetrahydrofuran; at 0 - 25 ℃; for 20h;
|
93% |
|
With sodium hydride; In N,N-dimethyl-formamide; for 5h;
|
92% |
|
With sodium hydride; In N,N-dimethyl-formamide; at 0 - 20 ℃; for 6h;
|
92% |
|
With sodium hydride; In N,N-dimethyl-formamide;
|
89% |
|
With sodium hydride;
|
86% |
|
With potassium hydroxide; 18-crown-6 ether; In tetrahydrofuran; for 0.25h; Ambient temperature;
|
85% |
|
With potassium hydroxide; 18-crown-6 ether; In tetrahydrofuran; at 20 ℃; for 3h;
|
85% |
|
|
83% |
|
With sodium hydride; In N,N-dimethyl-formamide; paraffin oil; at 0 - 20 ℃;
|
83% |
|
1,2:5,6-di-O-isopropylidene-α-D-glucofuranose; With tetrabutylammomium bromide; sodium hydroxide; In dichloromethane; at 0 - 20 ℃;
benzyl bromide; In dichloromethane; at 20 ℃;
|
80% |
|
With sodium hydroxide; tetrabutylammomium bromide; In dichloromethane; at 20 ℃; for 12h;
|
78% |
|
With sodium hydride; In tetrahydrofuran;
|
75% |
|
With tetra-(n-butyl)ammonium iodide; sodium hydride; In tetrahydrofuran; for 0.75h; Heating;
|
|
|
With sodium hydride; Yield given. Multistep reaction; 1.) DMF, 0 deg C, 30 min, 2.) DMF, RT, 30 min;
|
|
|
With sodium hydride; N,N-dimethyl-formamide; Multistep reaction; 1.) mineral oil, rt, 1 h 2.) rt, 3 h;
|
|
|
With Lithium dimsyl; Yield given. Multistep reaction; 1) DMSO, 2 h, 2) overnight;
|
|
|
With tetra-(n-butyl)ammonium iodide; sodium hydride; Multistep reaction; 1.) THF, from 0 deg C to RT, 2.) THF, 50 deg C, 2 h;
|
|
|
With sodium hydride; In tetrahydrofuran; N,N-dimethyl-formamide; Yield given;
|
|
|
With sodium hydride; In tetrahydrofuran; N,N-dimethyl-formamide;
|
|
|
With potassium hydroxide; at 130 ℃; for 4h;
|
|
|
With sodium hydride; Yield given. Multistep reaction; 1.) THF, 30 min, 2.) THF, DMF, room temperature, 2 h;
|
|
|
With tetra-(n-butyl)ammonium iodide; sodium hydride; In tetrahydrofuran; for 48h; Ambient temperature;
|
|
|
With sodium hydride; In N,N-dimethyl-formamide; at 20 ℃; for 2h;
|
|
|
With tetra-(n-butyl)ammonium iodide; sodium hydride; In tetrahydrofuran; at 20 ℃; for 14h;
|
|
|
With sodium hydride; In N,N-dimethyl-formamide; at 90 ℃; for 2h;
|
|
|
With tetra-(n-butyl)ammonium iodide; sodium hydride; In tetrahydrofuran; at 20 ℃; for 6h;
|
|
|
With tetra-(n-butyl)ammonium iodide; sodium hydride; In tetrahydrofuran; at 20 ℃; for 24h;
|
|
|
1,2:5,6-di-O-isopropylidene-α-D-glucofuranose; With sodium hydride; In tetrahydrofuran;
benzyl bromide; With tetra-(n-butyl)ammonium iodide; In tetrahydrofuran; at 20 ℃; for 10h; Further stages.;
|
|
|
With tetra-(n-butyl)ammonium iodide; sodium hydride; In tetrahydrofuran;
|
|
|
With sodium hydroxide; tetrabutylammomium bromide; In dichloromethane; water; at 20 ℃; for 16h;
|
|
|
With sodium hydride; In N,N-dimethyl-formamide; at 20 ℃; for 1h;
|
|
|
1,2:5,6-di-O-isopropylidene-α-D-glucofuranose; With sodium hydride; In N,N-dimethyl-formamide; at 0 ℃; for 0.5h;
benzyl bromide; In N,N-dimethyl-formamide; at 0 ℃; for 3h;
|
|
|
1,2:5,6-di-O-isopropylidene-α-D-glucofuranose; With sodium hydride; In N,N-dimethyl-formamide; mineral oil; at 0 ℃; Inert atmosphere;
benzyl bromide; In methanol; N,N-dimethyl-formamide; mineral oil; at 90 ℃; Inert atmosphere;
|
|
|
With sodium hydride; In tetrahydrofuran;
|
|
|
With sodium hydride; In N,N-dimethyl-formamide;
|
|
|
1,2:5,6-di-O-isopropylidene-α-D-glucofuranose; With sodium hydride; In tetrahydrofuran; at 0 ℃; for 0.333333h; Inert atmosphere;
benzyl bromide; With tetra-(n-butyl)ammonium iodide; In tetrahydrofuran; at 20 ℃; for 2h; Reflux;
|
|
|
With tetra-(n-butyl)ammonium iodide; In N,N-dimethyl-formamide; at 20 ℃; for 8h;
|
|
|
With tetra-(n-butyl)ammonium iodide; sodium hydride; In tetrahydrofuran; at 70 ℃; for 10h;
|
|
|
With sodium hydride; In tetrahydrofuran; at 0 - 20 ℃; for 8h; Inert atmosphere;
|
|
|
With tetra-(n-butyl)ammonium iodide; sodium hydride; In tetrahydrofuran; at 70 ℃; for 10h;
|
|
|
With sodium hydride; In tetrahydrofuran; Inert atmosphere; Schlenk technique;
|
|
|
1,2:5,6-di-O-isopropylidene-α-D-glucofuranose; With sodium hydride; In tetrahydrofuran; at 0 - 10 ℃; for 5h; Inert atmosphere;
benzyl bromide; With tetrabutylammomium bromide; In tetrahydrofuran; at 0 - 20 ℃;
|
|
|
1,2:5,6-di-O-isopropylidene-α-D-glucofuranose; With sodium hydride; In tetrahydrofuran; mineral oil; at 0 - 5 ℃; for 5h; Inert atmosphere;
benzyl bromide; With tetrabutylammomium bromide; In tetrahydrofuran; at 0 - 20 ℃; Inert atmosphere;
|
|
|
With tetra-(n-butyl)ammonium iodide; sodium hydride; In tetrahydrofuran; at 50 ℃;
|
|
|
With sodium hydride; at 20 ℃; Inert atmosphere;
|
|
|
1,2:5,6-di-O-isopropylidene-α-D-glucofuranose; With sodium hydride; In N,N-dimethyl-formamide; mineral oil; at 0 ℃; for 0.5h; Inert atmosphere;
benzyl bromide; In N,N-dimethyl-formamide; mineral oil; at 20 ℃; for 14h; Inert atmosphere;
|
|
|
1,2:5,6-di-O-isopropylidene-α-D-glucofuranose; With sodium hydride; In N,N-dimethyl-formamide; at 0 ℃; Inert atmosphere;
benzyl bromide; In N,N-dimethyl-formamide; at 0 - 20 ℃; for 2h; Inert atmosphere;
|
benzyl bromide
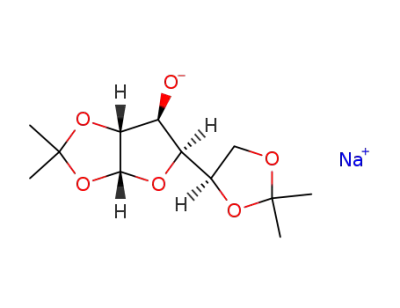
Natrium-Verbindung der O1,O2;O5,O6-Diisopropyliden-α-D-glucofuranose

benzyl chloride
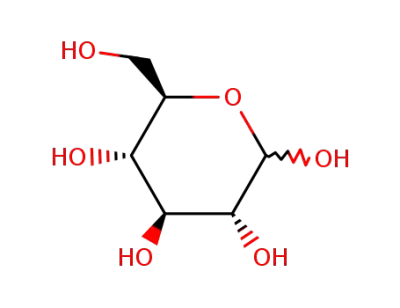
D-Glucose
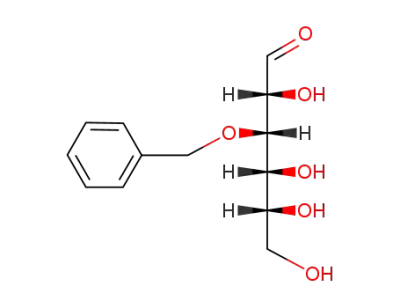
O3-benzyl-D-glucose
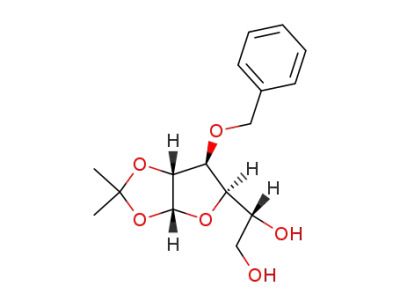
(1R)-1-((3aR,6S,6aR)-6-(benzyloxy)-2,2-dimethyltetrahydrofuro(2,3-d)(1,3)dioxol-5-yl)ethane-1,2-diol
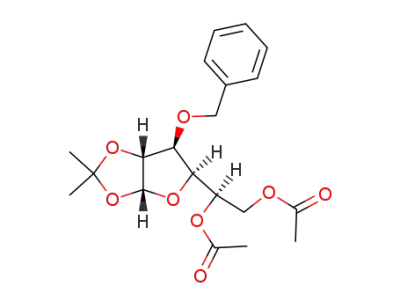
3-O-benzyl-1,2-O-isopropylidene-α-D-glucofuranose diacetate
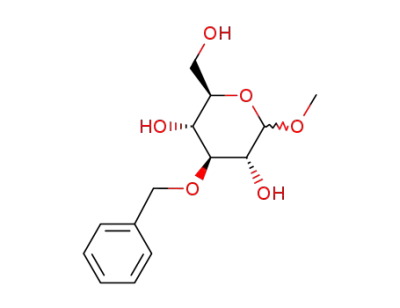
methyl 3-O-benzyl-α,β-D-glucopyranoside