Your Location:Home > Products > Medical intermediate > Methyl-α-D-Mannopyranoside
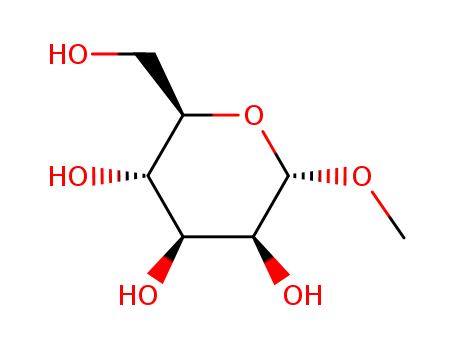

CasNo: 617-04-9
MF: C7H14O6
Appearance: White powder
Packing: 25kg/drum or as customer require.
Synonyms:alpha-Methyl-D-mannoside;1-O-Methyl-alpha-D-mannopyranoside; Methyl alpha-D-mannoside (VAN); NSC 1225; alpha-D-Methyl mannoside; alpha-Methyl mannopyranoside; alpha-Methyl-D-(-)-mannoside;
CAS Number: 617-04-9
EINECS No.: 210-502-3
Molecular Structure:
Molecular Formula: C7H14O6
Molecular Weight:194.18
Storage: Maintain in cool, dry containment.
Package: 25kg/drum or as customer require.
Expiration Date: 1 years.
Quality Standard:
|
Item |
Specification |
|
Appearance |
White to off-white powder |
|
Assay (GC), % |
≥98.0 |
|
Specific rotation, [α]20D(C=1,H2O)
|
+77.0~+81.0 |
|
Loss on drying, % |
≤0.5 |
1.It can be used as a plant growth agent, diluted and sprayed on radish leaves, outdoor lawns or other plants to improve the growth and development of plants.
2.Synthesis of methy 2,3,4,6-tetra-O-benzyl-α-D-mannopyranoside, and then synthesis of pharmaceutical intermediate such as 2,3,4,6-tetra-O-benzyl-α-D-mannopyranose.
3.Synthesis of mannopyranoside esters as antimicrobial agents.
4.Selective synthesis of 4,6-O-benzylidene of methyl α-D-mannopyranoside with 2,6-dimethylbenzaldehyde.
5.Surface engineering of solid lipid nanopatticle assemblies for the active targeting to macrophages in anti-tuberculosis inhalation.
6. Partial neopentyl acylation, intramolecular migration and enzymolysis can be performed.
7.Reactivity to release microencapsulated glucoamylase.
8. Synthesis of novel σ-receptor ligands.
9. It forms a sodium vanadate (V) complexes with methyl α- and β-D - galactopyranoside, and select O-methyl derivatives: a 51V and 13C NMR study.
10.Synthesis of L-chiro-inositol-1,2,3-trisphosphate and -1,2,3,5-tetrakisphosphate by ferrier reaction.
11. Synthesis of 2,3-di-O-glycosyl derivatives with methyl α-L-rhamnopyranoside.
12. Partial tosylation of methyl α-D-mannopyranoside.
13.Interaction of vicia faba lectin and methyl α-D-mannopyranoside was investigated by ultraviolet difference spectroscopy. The capacity of lectins to bind specifically to various carbohydrates makes them useful for the isolation and structural analysis of such glycoconjugates as membrane receptors .
14.Partial methylated derivatives were prepared with methyl α-D-galactopyranoside.
15. Chiral building blocks with methyl α-D - glucopyranoside for anthracyclinone synthesis.
16. Synthesis of some dialkjznylidene derivatives.
17. Synthesis of N-acylated 7-amino-2,6,7-trideoxy-D-erythroheptopyranosides .
18. Synthesis of methyl α-D -rhamnopyranoside.
19. For biochemical research. An eluent for the dissociation of glycoprotein complex with concomitant globulin A by affinity chromatography.
Aggregation facilitates analysis: The Ca...
Antifreeze proteins and glycoproteins [A...
Drug-resistant bacteria are still emergi...
A rapid, simple, and efficient deprotect...
Acetylated oligosaccharides are common i...
Novel antimicroalgal substances halymeci...
Five fucosylated glycoclusters exhibitin...
Traditional approaches to stereoselectiv...
Human glycans are primarily composed of ...
The selective manipulation of carbohydra...
The invention provides a carbon glycosid...
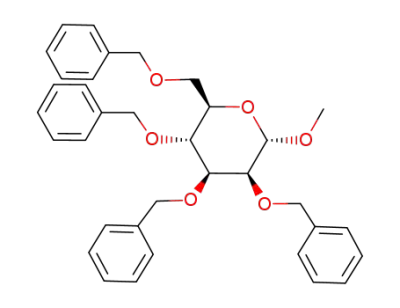
(2R,3R,4S,5S,6S)-3,4,5-tris(benzyloxy)-2-((benzyloxy)methyl)-6-methoxytetrahydro-2H-pyran

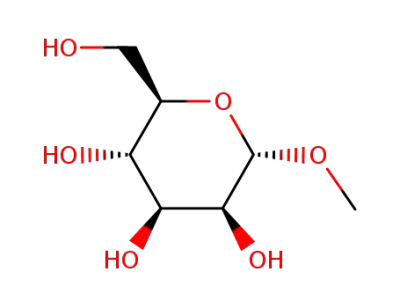
(2R,3S,4S,5S,6S)-2-(hydroxymethyl)-6-methoxytetrahydro-2H-pyran-3,4,5-triol
| Conditions | Yield |
|---|---|
|
With palladium 10% on activated carbon; hydrogen; In methanol; ethyl acetate; at 80 ℃; under 30003 Torr; Continuous flow hydrogenotion reactor;
|
95% |
|
With iron(III) chloride; In dichloromethane; for 0.5h; Ambient temperature;
|
71% |
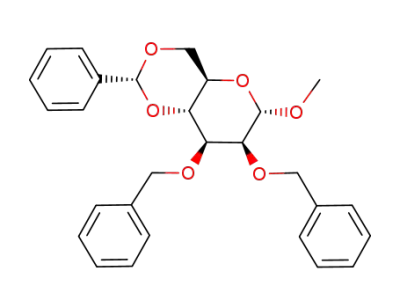
methyl 2,3-di-O-benzyl-4,6-O-benzylidene-α-D-mannopyranoside


(2R,3S,4S,5S,6S)-2-(hydroxymethyl)-6-methoxytetrahydro-2H-pyran-3,4,5-triol
| Conditions | Yield |
|---|---|
|
With palladium 10% on activated carbon; hydrogen; In methanol; ethyl acetate; at 80 ℃; under 30003 Torr; Continuous flow hydrogenotion reactor;
|
90% |
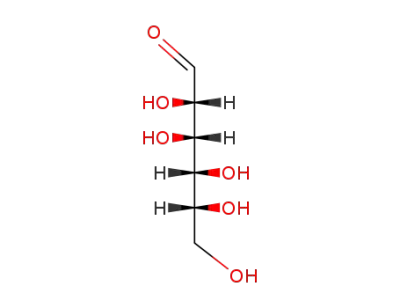
D-Mannose
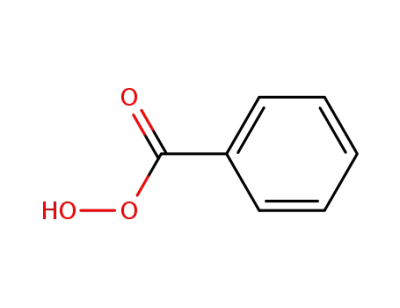
Perbenzoic acid
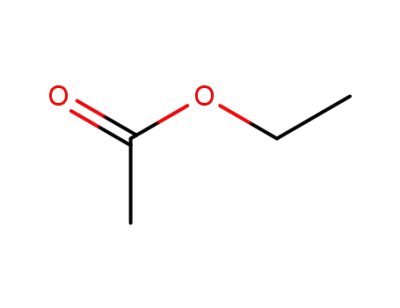
ethyl acetate
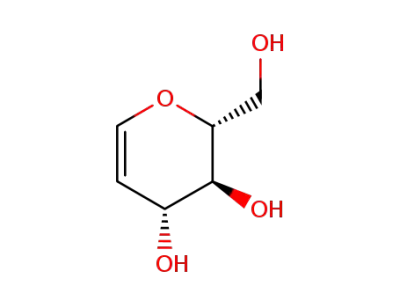
D-glucal
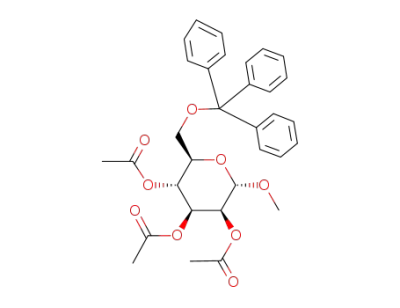
(2S,3S,4S,5R,6R)-2-methoxy-6-((trityloxy)methyl)tetrahydro-2H-pyran-3,4,5-triyl triacetate
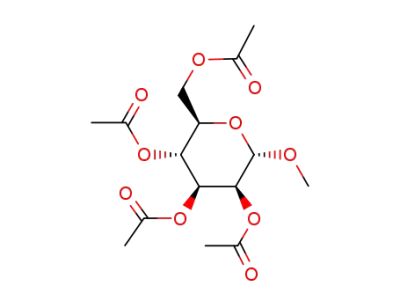
methyl 2,3,4,6-tetra-O-acetyl-α-D-mannopyranoside
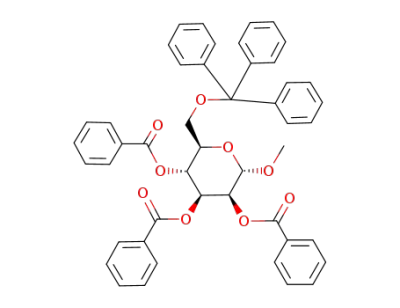
methyl 2,3,4-tri-O-benzoyl-6-O-trityl-α-D-mannopyranoside
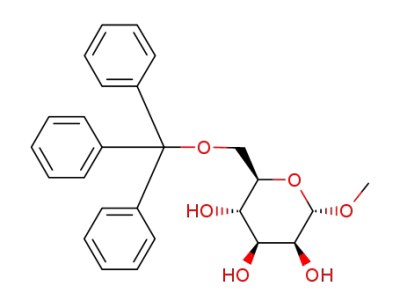
methyl 6-O-trityl-α-D-mannopyranoside