Your Location:Home > Products > Medical intermediate > 1,2-O-Isopropylidene-α-D-Glucurono-6,3-Lactone
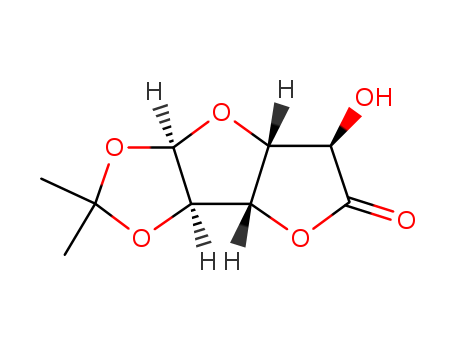

CasNo: 20513-98-8
MF: C9H12O6
Synonyms: 1,2-O-Isopropylidene-α-D-glucofuranurono-6,3-lactone;D-Glucorono-6,3-lactone acetonide;
Molecular Formula: C9H12O6
Molecular Weight: 216.19
CAS Number:20513-98-8
Molecular Structure:
.png)
|
Item |
Specification |
|
Appearance |
White to off-white crystalline powder |
|
Assay (GC),% |
≥98.0 |
|
Melting Point, ℃ |
119.0~123.0 |
|
Loss on drying,% |
≤0.5 |
|
Residue on ignition,% |
≤0.10 |
Glycoside pharmaceutical intermediates
Attempted synthesis of the imidazylate d...
The products (1) from the periodate oxid...
-
A novel, practical and concise synthesis...
2′-O,4′-C-Ethylene-bridged nucleic acid ...
The invention relates to the technical f...
The present invention relates to novel i...
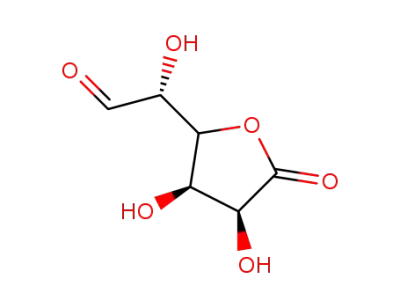
D-glucurono-6,3-lactone

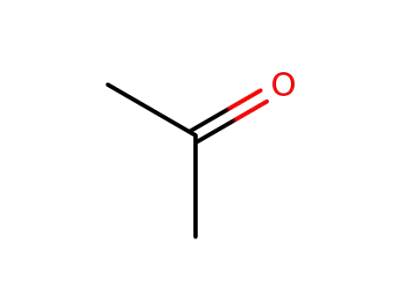
acetone

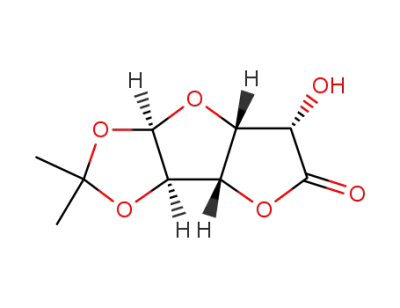
D-glucurono-3,6-lactone acetonide
| Conditions | Yield |
|---|---|
|
D-glucurono-6,3-lactone; acetone; In acetonitrile; at 50 ℃; for 0.5h; Molecular sieve;
With sulfuric acid; for 8h; Reflux;
|
90% |
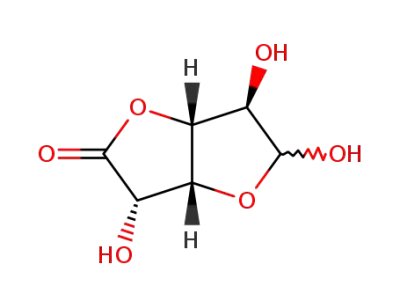
D-glucurono-6,3-lactone


acetone


D-glucurono-3,6-lactone acetonide
| Conditions | Yield |
|---|---|
|
With sulfuric acid;
|
93% |
|
With sulfuric acid; for 5h;
|
90% |
|
With sulfuric acid; at 20 ℃; for 4h; Inert atmosphere;
|
90% |
|
With toluene-4-sulfonic acid; copper(II) sulfate;
|
87% |
|
With sulfuric acid; at 20 ℃; for 38h;
|
84% |
|
With sulfuric acid;
|
82% |
|
With sulfuric acid; at 20 ℃; for 5h;
|
79% |
|
With acid;
|
78% |
|
With sulfuric acid;
|
|
|
sulfuric acid; In acetonitrile; at 25 ℃; for 18h; Heating / reflux;
|
|
|
With sulfuric acid; at 20 ℃;
|
|
|
With sulfuric acid; In acetonitrile; at 65 ℃;
|
|
|
With sulfuric acid;
|
100 mg |
|
With sulfuric acid; at 10 - 35 ℃; Inert atmosphere;
|
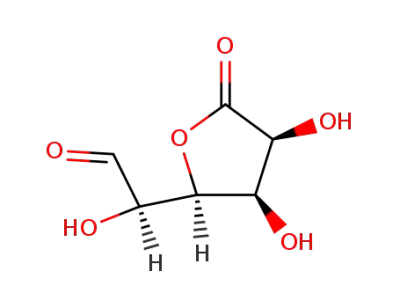
D-glucurono-6,3-lactone
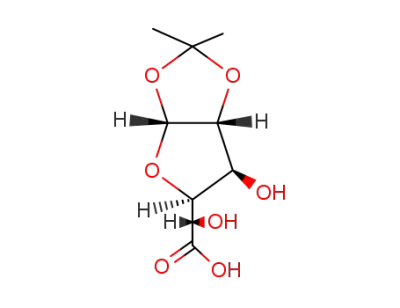
O1,O2-isopropylidene-α-D-glucofuranuronic acid
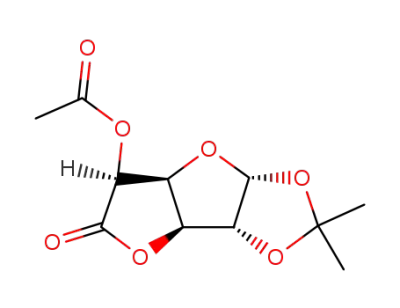
5-O-acetyl-1,2-O-isopropylidene-β-L-idofuranurono-6,3-lactone
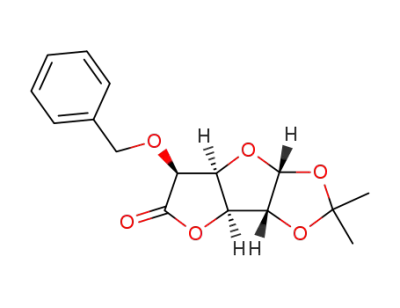
(1S,3R,4R,5S,8S)-8-benzyloxy-7-keto-3,4-isopropylidenedioxy-2,6-dioxabicyclo[3.3.0]octane
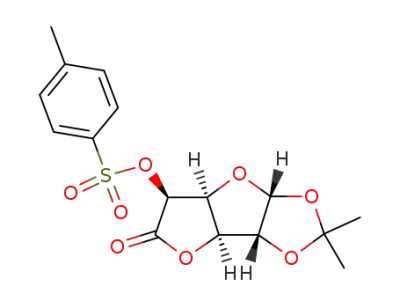
1,2-O-isopropylidene-5-O-toluene-p-sulphonyl-α-D-glucofuranurono-6,3-lactone
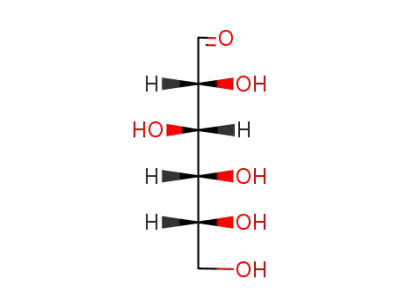
D-glucose
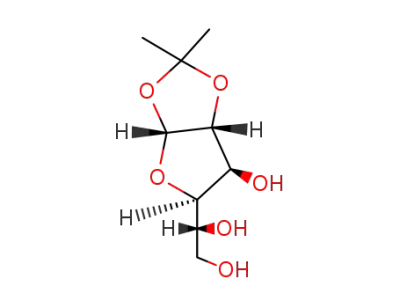
1,2-O-isopropylidene-α-D-glucofuranose
methyl 3,5-O-benzylidene-1,2-O-isopropylidene-α-D-glucofuranuronate