Your Location:Home > Products > Medical intermediate > 2',3'-O-Isopropylideneuridine
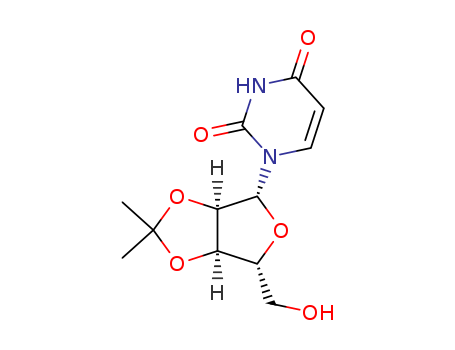

CasNo: 362-43-6
MF: C12H16N2O6
Packing: 25kg/drum or as required by customer
Synonyms: Isopropylideneuridine;2',3'-O-Isopropylidene-D-uridine;Uridine,2',3'-O-(1-methylethylidene)-;Uridine,2',3'-O-isopropylidene;Uridine 2',3'-acetonide
Molecular Formula: C12H16N2O6
Molecular Weight: 284.27
CAS Number:362-43-6
Molecular Structure:
.png)
|
Item |
Specification |
|
Appearance |
White to off-white crystalline powder |
|
Assay (HPLC),% |
≥98.0 |
|
Melting Point, ℃ |
163.0~168.0 |
|
Loss on drying,% |
≤1.0 |
|
Residue on ignition,% |
≤0.10 |
Characteristics:It is white to off-white crystalline powder. It is easily soluble in water and slightly soluble in methanol.
Monupivir (Molnupiravir) is a broad-spectrum anti-RNA virus drug, which has obvious effect on novel coronavirus (COVID-19),significant inhibitory effect on the proliferation of SARS-CoV-2 virus. It is the first oral anti-novel coronavirus drug in the world, which can reduce the risk of hospitalization or death by 50% of COVID-19 patients. 2 ′, 3 ′ -O-isoisopropyl uridine is an important intermediate in Molnupiravir synthesis.
Storage: Store in a tightly closed container. Maintain in a cool and dry area.
Package: 25kg/drum or as required by customer.
Expiration Date: 2 years
Nucleoside phosphonates have been design...
Novel hybrid porphyrins bearing two and ...
A new type flexible receptor which has t...
Protein O-linked β-D-N-acetylglucosamine...
The preparation of natural product-inspi...
2',3'-O-Isopropylideneuridine (4) was pr...
The 5′-alkynylation of uridine-derived a...
Reported is an efficient synthesis of ad...
Despite being in routine for onco-diagno...
High-throughput small-molecule screening...
The photophysical properties of the conj...
-
Quaternary ammonium salts are a group of...
Phosphoglycosyltransferases (PGTs) repre...
A regioselective synthesis of 6-ω-alkeny...
Phosphoramidates 1 and 2 were synthesize...
Orotidine 5′-monophosphate decarboxylase...
In this paper, we report that a versatil...
This paper reports a simple method for t...
The synthesis of several C60 derivatives...
The uridyl peptide antibiotics (UPAs), o...
Copper-catalyzed azide-alkyne cycloaddit...
The LDA-induced coupling of 2′,3′,5′-O-p...
Sugar-modified nucleosides are prime syn...
The critical role of sialyltransferase (...
A new nucleoside modified by prenylation...
A library of novel L-propargylglycine-ba...
Herein, we report the design and synthes...
The O-linked β-N-acetylglucosamine (O-Gl...
Herein is described the development of a...
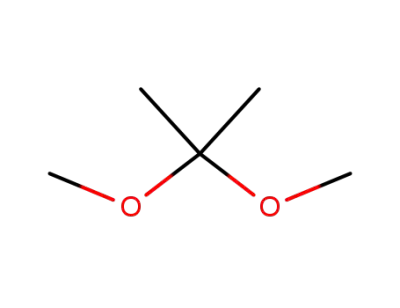
2,2-dimethoxy-propane

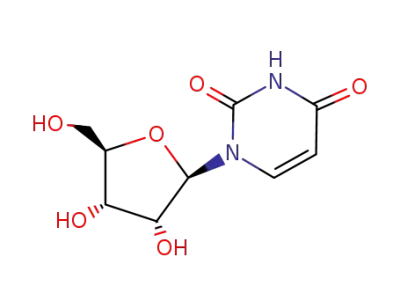
uridine

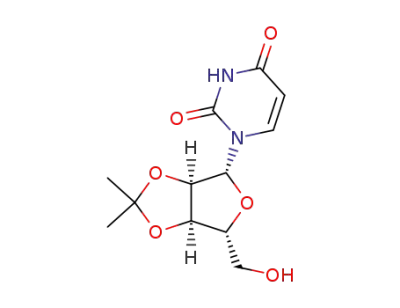
2',3'-O-isopropylideneuridine
| Conditions | Yield |
|---|---|
|
With toluene-4-sulfonic acid; In acetone; for 1h; Reflux;
|
100% |
|
With toluene-4-sulfonic acid; In acetone; for 4h; Reflux;
|
100% |
|
With toluene-4-sulfonic acid; In acetone; for 1h; Reflux;
|
99% |
|
With toluene-4-sulfonic acid; In acetone; at 60 ℃; for 24h; Inert atmosphere;
|
96% |
|
With toluene-4-sulfonic acid; In acetone; at 60 ℃; for 24h;
|
96% |
|
With toluene-4-sulfonic acid; In acetonitrile; for 3h; Inert atmosphere; Reflux;
|
95% |
|
With toluene-4-sulfonic acid; In acetonitrile; for 3h; Reflux;
|
94% |
|
With toluene-4-sulfonic acid; In acetonitrile; for 3h; Reflux;
|
94% |
|
With toluene-4-sulfonic acid; In acetone; at 20 ℃; for 2h;
|
93% |
|
With sulfuric acid; In acetone; at 0 - 55 ℃; for 8.5h; Time; Industrial scale;
|
91% |
|
With toluene-4-sulfonic acid; In N,N-dimethyl-formamide;
|
90% |
|
With toluene-4-sulfonic acid; In acetone; at 20 ℃; for 1.5h; Inert atmosphere;
|
90% |
|
With toluene-4-sulfonic acid; In acetone; at 20 ℃; Inert atmosphere;
|
88% |
|
With toluene-4-sulfonic acid; at 20 ℃; for 3h;
|
85% |
|
With toluene-4-sulfonic acid; In water; acetone; at 60 ℃; for 2h; Inert atmosphere;
|
81% |
|
With toluene-4-sulfonic acid; In acetone; at 60 ℃; for 2h; Inert atmosphere;
|
81% |
|
With toluene-4-sulfonic acid; In acetone; at 20 ℃;
|
80% |
|
|
79% |
|
With toluene-4-sulfonic acid; In acetone; for 24h; Reflux; Inert atmosphere;
|
79% |
|
With toluene-4-sulfonic acid; In acetone;
|
73% |
|
With toluene-4-sulfonic acid; In acetone; at 0 ℃; for 1h; Reflux; Inert atmosphere;
|
65% |
|
With toluene-4-sulfonic acid; In N,N-dimethyl-formamide; at 20 ℃; for 18h;
|
61% |
|
With DOWEX (50WX8) ion exchange resin (H+); In acetone;
|
58% |
|
With toluene-4-sulfonic acid; In N,N-dimethyl-formamide; at 40 ℃; for 3h; Molecular sieve;
|
56% |
|
With toluene-4-sulfonic acid; In N,N-dimethyl-formamide; at 40 ℃; for 1.5h; Inert atmosphere; Molecular sieve;
|
24.8% |
|
With toluene-4-sulfonic acid;
|
|
|
With toluene-4-sulfonic acid; In tetrahydrofuran;
|
|
|
2,2-dimethoxy-propane; uridine; With toluene-4-sulfonic acid; In water; N,N-dimethyl-formamide; at 45 ℃; for 2h; Molecular sieve;
With Amberlyst A-21 resin; In water; N,N-dimethyl-formamide; at 20 ℃; for 0.333333h;
|
|
|
With sulfuric acid; In acetone; Inert atmosphere;
|
45 g |
|
With toluene-4-sulfonic acid; In N,N-dimethyl-formamide;
|
|
|
2,2-dimethoxy-propane; uridine; In acetone; for 0.166667h;
With sulfuric acid; In acetone; at 20 ℃; for 0.5h;
|
![3-((benzyloxy)methyl)-1-((3aR,4R,6R,6aR)-6-(hydroxymethyl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)pyrimidine-2,4(1H,3H)-dione](/upload/2026/1/27b8f872-9c86-4ef9-b6f6-58f82f1e59b8.png)
3-((benzyloxy)methyl)-1-((3aR,4R,6R,6aR)-6-(hydroxymethyl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)pyrimidine-2,4(1H,3H)-dione


2',3'-O-isopropylideneuridine
| Conditions | Yield |
|---|---|
|
With formic acid; palladium on activated charcoal; hydrogen; In water; isopropyl alcohol; for 6h; under 760.051 Torr;
|
99% |
acetone

uridine
5-methoxy-N-acetyl-tryptamine
2',3'-O-isopropylidene-5-bromouridine
2'-3'-O-isopropylideneuridine 5'-(bis(p-nitrophenyl)phosphate)
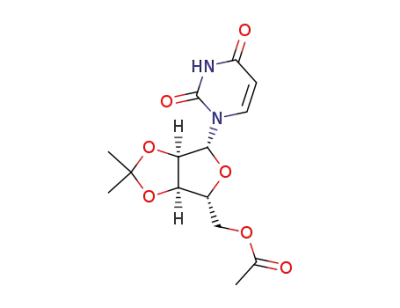
1-(5-O-Acetyl-2,3-O-isopropylidene-β-D-ribofuranosyl)uracil
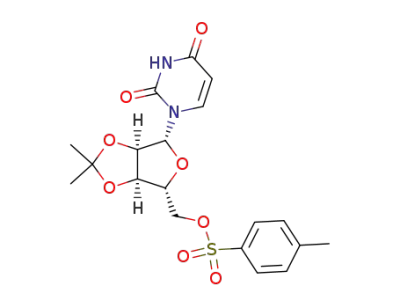
2',3'-O-isopropylidene-5'-O-tosyluridine
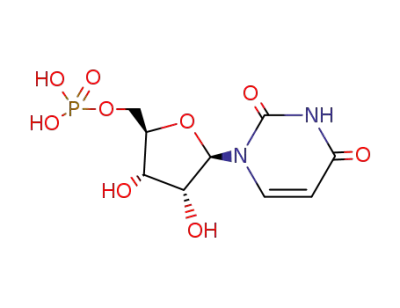
5'-Uridylic Acid