Your Location:Home > Products > Medical intermediate > 3-O-Benzyl-1,2:5,6-Di-O-Isopropylidene-α-D-Allofuranose
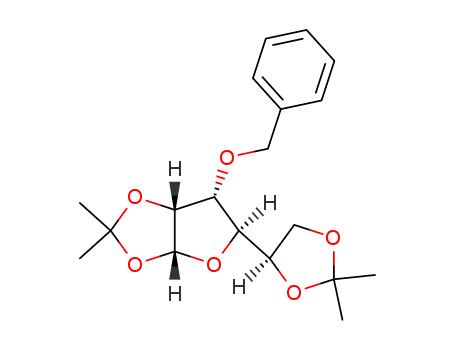

CasNo: 22331-21-1
MF: C19H26 O6
Packing: 25kg/drum or as required by customer
Synonyms: 1,2:5,6-di-O-isopropylidene-3-O-benzyl-α-D-allofuranose;
Molecular Formula: C19H26O6
Molecular Weight: 350.41
CAS Number:22331-21-1
Molecular Structure:
.png)
|
Item |
Specification |
|
Appearance |
White to off-white crystalline powder |
|
Assay (GC),% |
≥98.0 |
|
Melting Point, ℃ |
62.0~69.0 |
|
Specific rotation,(C=1,CHCl3) |
+106.0~ +111.0° |
|
Loss on drying,% |
≤0.5 |
|
Residue on ignition,% |
≤0.10 |
Characteristics: It is white to off-white crystalline powder. It is easily soluble in cyclohexane and very slightly soluble in water.
3-O-Benzyl-1,2:5,6-di-O-isopropylidene-a-D-allofuranose is used in the synthesis of complex carbohydrates or as a sugar substitute,such as 4-C-cyclopropyl-D-ribo-tetrofuranose derivatives、5,6-dideoxy-3-O-methyl-5-C-(phenylphosphinyl)-D-glucopyranose、aristeromycin from D-glucose and so on.
Storage: Store in a tightly closed container. Maintain in a cool and dry area.
Package: 25kg/drum or as required by customer.
Expiration Date: 2 years
6-Methylpurine (MeP) is cytotoxic adenin...
A study of the potential use of 5-exo fr...
The present invention relates to the man...
The present disclosure relates, in gener...
Comparative genomics of the bacterial th...
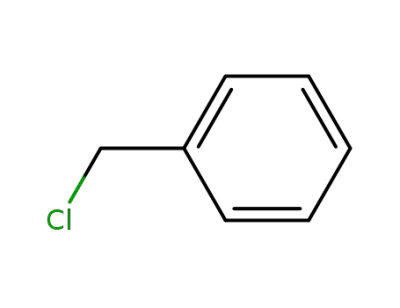
benzyl chloride

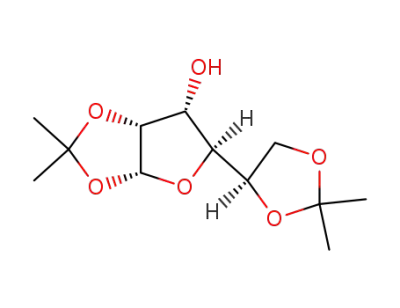
Diacetone D-glucose

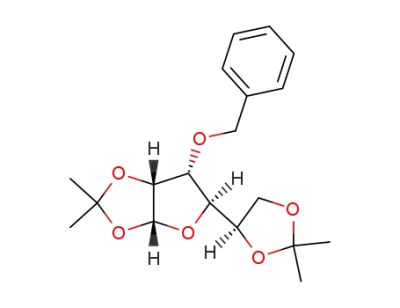
1,2:5,6-di-O-isopropylidene-3-O-benzyl-α-D-allofuranose
| Conditions | Yield |
|---|---|
|
With tetra(n-butyl)ammonium hydrogensulfate; sodium hydroxide; In toluene; at 30 - 90 ℃; for 4h; Large scale;
|
85% |
benzyl bromide


Diacetone D-glucose


1,2:5,6-di-O-isopropylidene-3-O-benzyl-α-D-allofuranose
| Conditions | Yield |
|---|---|
|
With sodium hydride; In N,N-dimethyl-formamide; at 20 ℃; for 8h;
|
100% |
|
Diacetone D-glucose; With sodium hydride; In acetonitrile; mineral oil; at 0 ℃; for 1h; Inert atmosphere;
benzyl bromide; In methanol; acetonitrile; mineral oil; at 0 ℃; for 4.5h;
|
90% |
|
With sodium hydride;
|
60% |
|
|
|
|
With sodium hydride; In N,N-dimethyl-formamide;
|
|
|
With N,N,N,N,N,N-hexamethylphosphoric triamide; sodium hydride; In tetrahydrofuran; Inert atmosphere;
|
|
|
With sodium hydride; In N,N-dimethyl-formamide;
|
|
|
Diacetone D-glucose; With sodium hydride; In N,N-dimethyl-formamide; at 0 - 18 ℃; for 1h;
benzyl bromide; In N,N-dimethyl-formamide; at 0 - 18 ℃;
|
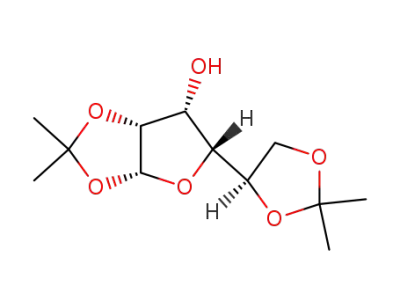
(1,2:5,6)-di-O-isopropylidene-D-gulose
benzyl bromide

benzyl chloride

Diacetone D-glucose
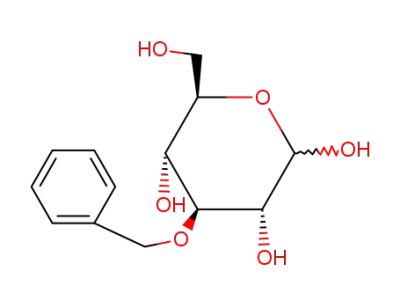
3-O-benzyl-D-glucopyranose
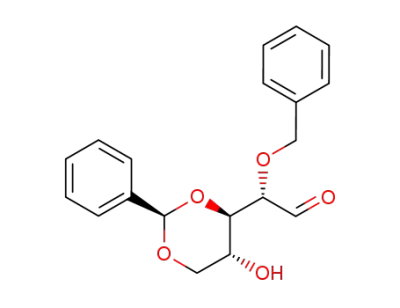
(S)-Benzyloxy-((2R,4R,5R)-5-hydroxy-2-phenyl-[1,3]dioxan-4-yl)-acetaldehyde
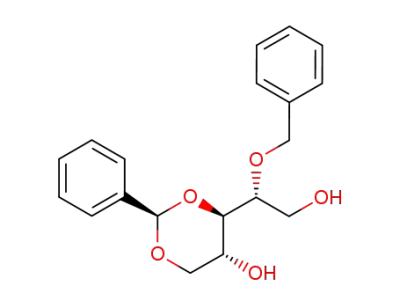
(2R,4R,5R)-4-((R)-1-Benzyloxy-2-hydroxy-ethyl)-2-phenyl-[1,3]dioxan-5-ol
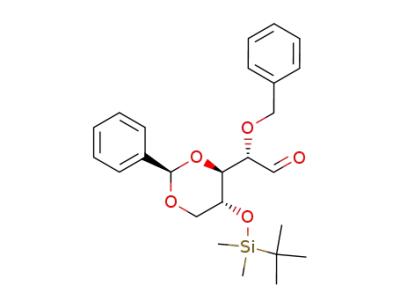
(S)-Benzyloxy-[(2R,4S,5R)-5-(tert-butyl-dimethyl-silanyloxy)-2-phenyl-[1,3]dioxan-4-yl]-acetaldehyde