Your Location:Home > Products > Medical intermediate > 2,3,4,6-Tetra-O-Benzyl-D-Glucono-1,5-Lactone

CasNo: 13096-62-3
MF: C34H34O6
Packing: 25kg/drum or as required by customer
Synonyms: 2,3,4,6-Tetra-O-benzyl-D-glucono-1,5-lactone;(3R,4S,5R,6R)-3,4,5-tris(benzyloxy)-6-((benzyloxy)methyl)tetrahydro-2H-pyran-2-one;
Molecular Formula: C34H34O6
Molecular Weight: 538.63
CAS Number:13096-62-3.png)
Specification
|
Item |
Specification |
|
Appearance |
light yellow to wine red clear liquid |
|
Assay (HPLC),% |
≥95.0 |
|
Residue on ignition,,% |
≤0.1% |
Characteristics: It is colorless to light yellow to wine red clear liquid. It is easily soluble in butanone and toluene and insoluble in water.
Gluconate lactone is the most common glucose derivative, widely used in industrial production and scientific research. It is widely used in the synthesis of drugs, drug intermediates, and natural products. It is one of the key raw materials for the synthesis of voglibose and spironolactone sugars. Gluconate lactones can also be used to synthesize C-glycosides that do not have carbon carbon double bond connections in nature; Synthesize sugar drugs Neu5Ac and Voglibose, and react with Grignard reagents or organic lithium for the synthesis of sugar heterocyclic carbon chains, spirocyclic compounds, nitrogen heterosaccharides, and polymer monomer compounds, with a wide range of applications.
Storage: Maintain in a cool and dry area.
Package: 25kg/drum or as required by customer.
Expiration Date: 2 years
Nickel- and palladium-catalyzed Fukuyama...
2,3,4,6-Tetra-O-benzyl-D-glucopyranose, ...
The present invention relates to glucopy...
The invention provides a synthesis metho...
The invention discloses a method for con...
The invention discloses a synthesis meth...
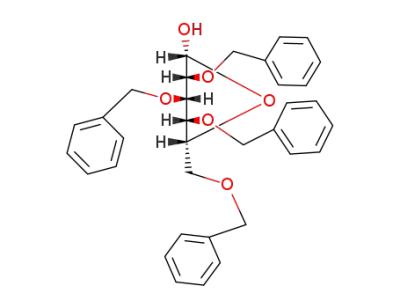
2,3,4,6-tetra-O-benzyl-D-glucopyranoside

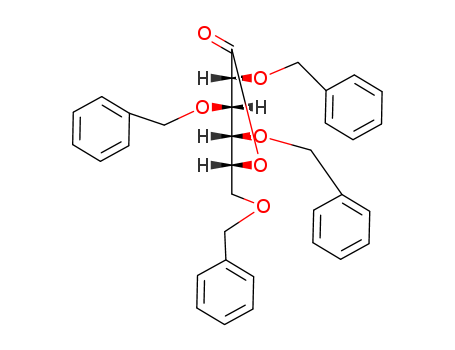
(3R,4S,5R,6R)-3,4,5-tris(benzyloxy)-6-((benzyloxy)methyl)tetrahydro-2H-pyran-2-one
| Conditions | Yield |
|---|---|
|
With acetic anhydride; dimethyl sulfoxide; at 20 ℃;
|
93% |
|
2,3,4,6-tetra-O-benzyl-D-glucopyranoside; In dimethyl sulfoxide; at 20 - 25 ℃; for 0.5h; Inert atmosphere;
With acetic anhydride; at 15 - 25 ℃; Inert atmosphere;
|
92% |
|
2,3,4,6-tetra-O-benzyl-D-glucopyranoside; With 4-methylmorpholine N-oxide; In dichloromethane; at 20 ℃; for 0.333333h; Molecular sieve;
With tetrapropylammonium perruthennate; In dichloromethane; at 20 ℃; for 2h;
|
82% |
|
2,3,4,6-tetra-O-benzyl-D-glucopyranoside; With oxalyl dichloride; dimethyl sulfoxide; In dichloromethane; at -78 ℃; for 105h;
With triethylamine; In dichloromethane; at -78 - 20 ℃; for 1h;
|
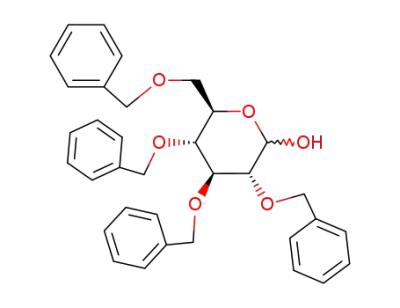
2,3,4,6-Tetra-O-benzyl-D-glucopyranose


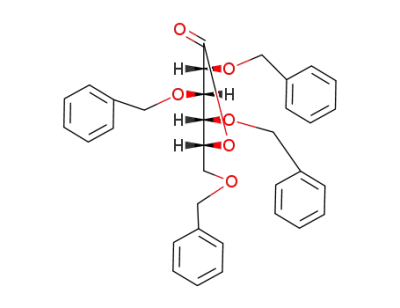
(3R,4S,5R,6R)-3,4,5-tris(benzyloxy)-6-((benzyloxy)methyl)tetrahydro-2H-pyran-2-one
| Conditions | Yield |
|---|---|
|
With sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium hydrogencarbonate; potassium bromide; In dichloromethane; water; at 0 ℃; for 0.333333h; Solvent;
|
100% |
|
With 2,2,6,6-tetramethyl-piperidine-N-oxyl; sodium hypochlorite; sodium hydrogencarbonate; potassium bromide; In dichloromethane; water; at 0 ℃; for 0.333333h;
|
100% |
|
With sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium hydrogencarbonate; potassium bromide; In dichloromethane; water; at 0 ℃; for 0.333333h;
|
100% |
|
With acetic anhydride; dimethyl sulfoxide; at 20 ℃; for 12h;
|
99% |
|
With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; N-methyl-N-[3-(4-diacetoxyiodo)phenoxy-1-propyl]pyrrolidinium 4-methylbenzenesulfonate; In dichloromethane; at 20 ℃; for 5h; Inert atmosphere;
|
99% |
|
With bromobenzene; potassium carbonate; triphenylphosphine; palladium diacetate; In tetrahydrofuran; for 4h; Heating;
|
98% |
|
With acetic anhydride; In dimethyl sulfoxide; at 20 ℃; for 10h;
|
98% |
|
With acetic anhydride; dimethyl sulfoxide; at 25 ℃; for 12h; Temperature; Time;
|
98.5% |
|
With pyridine; N-(2,2,6,6-tetramethyl-1-oxopiperidin-1-ium-4-yl)acetamide tetrafluoroborate; In dichloromethane; at 20 ℃;
|
97% |
|
With Iodine monochloride; caesium carbonate; In dichloromethane; at 0 - 20 ℃; for 3h; Reagent/catalyst; Solvent; Temperature; Green chemistry;
|
97% |
|
With acetic anhydride; dimethyl sulfoxide; at 22 ℃; for 18h;
|
96% |
|
With acetic anhydride; In dimethyl sulfoxide; at 20 ℃; for 16h; Inert atmosphere;
|
96% |
|
With dimethyl sulfoxide; In acetic anhydride; at 20 ℃; for 8h;
|
96% |
|
With acetic anhydride; In dimethyl sulfoxide; at 20 ℃; for 20h; Inert atmosphere; Schlenk technique; Glovebox;
|
96% |
|
With 3 A molecular sieve; pyridinium chlorochromate; In dichloromethane; for 0.75h;
|
95% |
|
With tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide; In acetonitrile; at 20 ℃; for 0.5h;
|
95% |
|
With dimethyl sulfoxide; In acetic anhydride;
|
95% |
|
With acetic anhydride; dimethyl sulfoxide; for 12h;
|
95% |
|
With tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide; In dichloromethane; at 20 ℃; Inert atmosphere; Molecular sieve;
|
95% |
|
With tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide; In dichloromethane; at 20 ℃; for 2h; Molecular sieve;
|
95% |
|
With acetic anhydride; In dimethyl sulfoxide; at 20 ℃;
|
95% |
|
With sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium hydrogencarbonate; acetic acid; potassium bromide; In dichloromethane; water; at 20 - 35 ℃;
|
95.98% |
|
With pyridinium chlorochromate; In dichloromethane;
|
95% |
|
|
94% |
|
With tetrapropylammonium perruthennate; 3 A molecular sieve; 4-methylmorpholine N-oxide; In dichloromethane; for 1h; Ambient temperature;
|
94% |
|
With tetrapropylammonium perruthennate; 3 A molecular sieve; 4-methylmorpholine N-oxide; In dichloromethane; for 1h; Product distribution; Ambient temperature; var. oxidants; var. solv., temp. and time;
|
94% |
|
With acetic anhydride; dimethyl sulfoxide;
|
94% |
|
With acetic anhydride; dimethyl sulfoxide; at 30 ℃; for 12h;
|
94% |
|
With acetic anhydride; dimethyl sulfoxide; at 20 ℃;
|
92% |
|
With acetic anhydride; dimethyl sulfoxide; at 20 ℃;
|
92% |
|
With acetic anhydride; dimethyl sulfoxide; at 20 ℃; for 18h; Inert atmosphere;
|
91% |
|
With Dess-Martin periodane; tert-butyl alcohol; In dichloromethane; at 25 ℃;
|
89% |
|
With acetic anhydride; In dimethyl sulfoxide; at 20 ℃; for 22h; Inert atmosphere;
|
89% |
|
With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; dihydrogen peroxide; sodium hydroxide; In water; at 65 ℃; for 0.166667h; Reagent/catalyst; Temperature;
|
88.1% |
|
With tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide; In dichloromethane; at 20 ℃; for 3.33333h; Inert atmosphere; Molecular sieve;
|
86% |
|
2,3,4,6-Tetra-O-benzyl-D-glucopyranose; With 4-methylmorpholine N-oxide; In dichloromethane; at 20 ℃; for 0.333333h; Inert atmosphere; Molecular sieve;
With tetra-n-propylammonium perruthenate.; In dichloromethane; at 20 ℃; for 3h; Inert atmosphere;
|
86% |
|
With acetic anhydride; dimethyl sulfoxide; Ambient temperature;
|
84% |
|
With 5-nitro-3-oxo-1λ3-benzo[d][1,2]iodaoxol-1(3H)-yl acetate; In N,N-dimethyl-formamide; at 65 ℃; for 24h;
|
84% |
|
With acetic anhydride; In dimethyl sulfoxide; at 20 ℃;
|
83% |
|
With acetic anhydride; In dimethyl sulfoxide; at 20 ℃; Inert atmosphere;
|
82% |
|
With acetic anhydride; dimethyl sulfoxide; In dimethyl sulfoxide; at 20 ℃; Inert atmosphere;
|
80% |
|
2,3,4,6-Tetra-O-benzyl-D-glucopyranose; With oxalyl dichloride; 3-methyl-1-[6-(methanesulfinyl)hexan-1-yl]-1H-imidazolium p-toluenesulfonate; In dichloromethane; at -70 ℃; for 0.5h;
With triethylamine; In dichloromethane; at -70 - 20 ℃; for 5.5h;
|
74% |
|
With iodine; potassium carbonate; In tert-butyl alcohol; at 90 ℃; for 10h; Inert atmosphere;
|
68% |
|
With acetic anhydride; dimethyl sulfoxide; at 20 ℃; Inert atmosphere;
|
67% |
|
With pyridinium chlorochromate; In dichloromethane; at 20 ℃; for 48h; Inert atmosphere; Molecular sieve;
|
57% |
|
With oxalyl dichloride; triethylamine; In dichloromethane; dimethyl sulfoxide; -78 deg C;
|
|
|
With acetic anhydride; dimethyl sulfoxide; Yield given;
|
|
|
With oxalyl dichloride; dimethyl sulfoxide; triethylamine; Yield given. Multistep reaction; 1a.) CH2Cl2, below -50, 90 min, 1b.) -40 deg C, 1c.) -10 deg C, 10 min, 1d.) -60 deg C, 2.) r.t, 1 h;
|
|
|
With 4 A molecular sieve; pyridinium chlorochromate; Yield given;
|
|
|
With pyridinium chlorochromate; In dichloromethane;
|
|
|
With dimethyl sulfoxide; In acetic anhydride;
|
|
|
With pyridinium chlorochromate; In dichloromethane; at 20 ℃; for 1h;
|
|
|
With molecular sieve; pyridinium chlorochromate; In dichloromethane;
|
|
|
With dimethyl sulfoxide; In acetic anhydride;
|
|
|
Multi-step reaction with 2 steps
1: 35 percent / triethylamine / tetrahydrofuran / 4 h / 0 °C
2: 60 percent / palladium acetate / acetonitrile / 2 h / 80 °C
With palladium diacetate; triethylamine; In tetrahydrofuran; acetonitrile;
|
|
|
With pyridinium chlorochromate; In dichloromethane; at 20 ℃; for 2h; Inert atmosphere; Molecular sieve;
|
|
|
With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium hydrogencarbonate; In dichloromethane;
|
|
|
2,3,4,6-Tetra-O-benzyl-D-glucopyranose; With oxalyl dichloride; dimethyl sulfoxide; In dichloromethane; at -78 ℃; for 0.25h;
With triethylamine; In dichloromethane; at -78 - 0 ℃;
|
3.2 g |
|
2,3,4,6-Tetra-O-benzyl-D-glucopyranose; With oxalyl dichloride; dimethyl sulfoxide; In dichloromethane; at -60 - -50 ℃; for 1h;
With triethylamine; In dichloromethane; at -50 - 20 ℃; for 1.16667h;
|
|
|
With 5-nitro-3-oxo-1λ3-benzo[d][1,2]iodaoxol-1(3H)-yl acetate; In N,N-dimethyl-formamide; at 60 ℃; for 24h; Reagent/catalyst;
|
425.2 mg |
|
With 5-nitro-3-oxo-1λ3-benzo[d][1,2]iodaoxol-1(3H)-yl acetate; In N,N-dimethyl-formamide; at 60 ℃; for 24h; Reagent/catalyst;
|
|
|
With oxalyl dichloride; dimethyl sulfoxide; triethylamine; In dichloromethane; at -78 ℃;
|
|
|
With acetic anhydride; sodium hydrogencarbonate; In dimethyl sulfoxide; at 0 - 20 ℃; for 16h;
|
|
|
With acetic anhydride; dimethyl sulfoxide; at 0 - 20 ℃; for 16h;
|
|
|
With acetic anhydride; In dimethyl sulfoxide; at 0 - 20 ℃; for 16h;
|
|
|
With acetic anhydride; In dimethyl sulfoxide; at 0 - 20 ℃; for 16h;
|
|
|
With acetic anhydride; In dimethyl sulfoxide; at 0 - 20 ℃; for 16h;
|
|
|
With tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide; In dichloromethane; at 20 ℃; for 2.33333h;
|
|
|
With acetic anhydride; In dimethyl sulfoxide; at 0 - 20 ℃; for 16h;
|
|
|
With acetic anhydride; In dimethyl sulfoxide; at 0 - 20 ℃; for 16h;
|
|
|
With acetic anhydride; In dimethyl sulfoxide; at 0 - 20 ℃; for 16h;
|
|
|
2,3,4,6-Tetra-O-benzyl-D-glucopyranose; With 2,2,6,6-tetramethyl-piperidine-N-oxyl; sodium hydrogencarbonate; potassium bromide; In dichloromethane; at 2 ℃; for 0.0833333h;
With sodium hypochlorite; In dichloromethane; water; for 0.5h;
|
105 g |
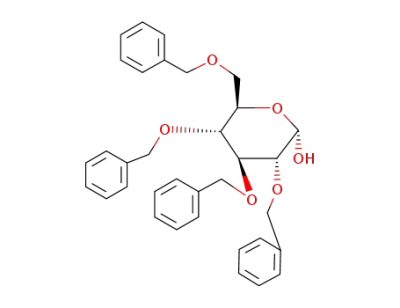
2,3,4,6-tetra-O-benzyl-D-glucopyranose

2,3,4,6-Tetra-O-benzyl-D-glucopyranose
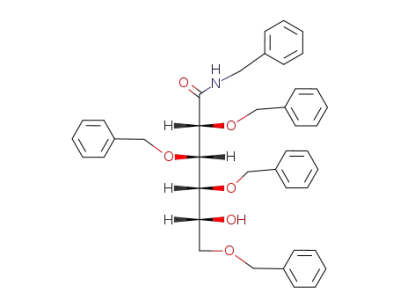
(2R,3S,4R,5R)-N-benzyl-5-hydroxy-2,3,4,6-tetrabenzyloxyhexanamide
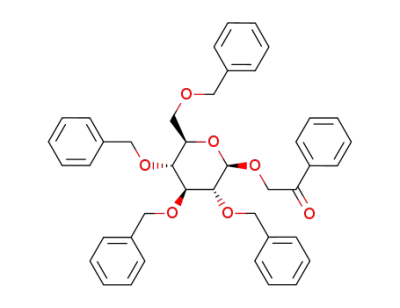
phenacyl 2,3,4,6-tetra-O-benzyl-β-D-glucopyranoside
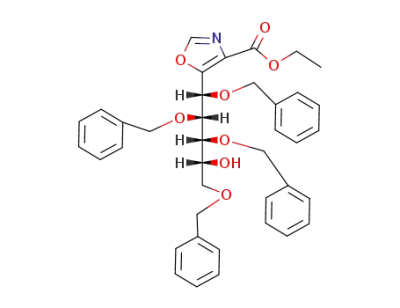
5-(Dr-1cF,2tF,3cF,5-tetrakis-benzyloxy-4rF-hydroxy-pent-catF-yl)-oxazole-4-carboxylic acid ethyl ester
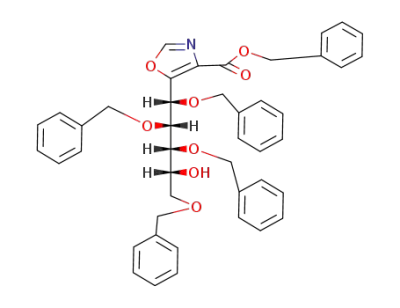
5-(Dr-1cF,2tF,3cF,5-tetrakis-benzyloxy-4rF-hydroxy-pent-catF-yl)-oxazole-4-carboxylic acid benzyl ester
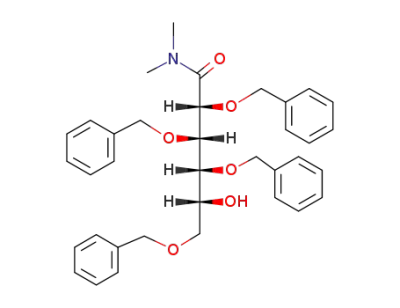
2,3,4,6-Tetra-O-benzyl-N,N-dimethyl-D-gluconamid
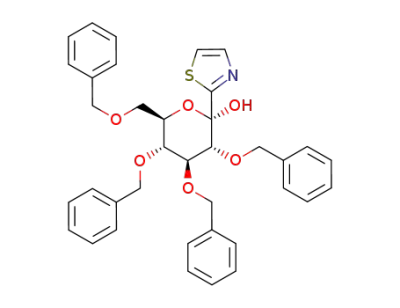
2,3,4,6-tetra-O-benzyl-β-1-C-(2-thiazolyl)-D-glucopyranose