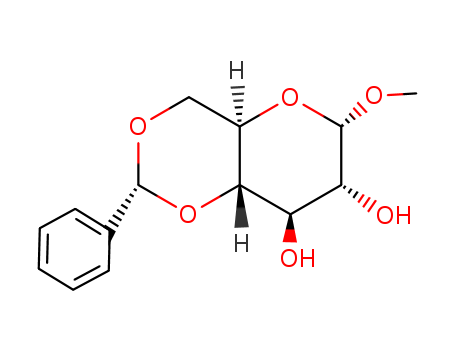
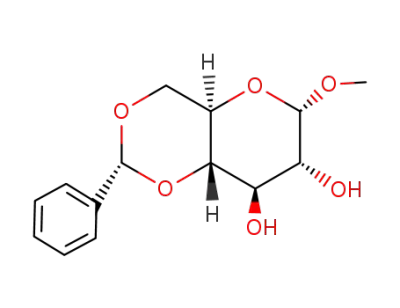
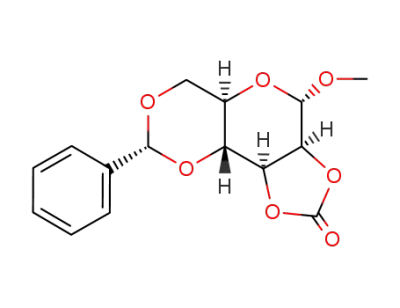
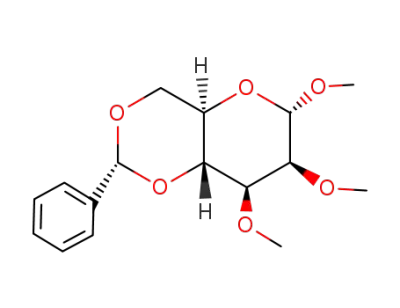
Methyl 4,6-O-Benzylidene-α-D-Glucopyranoside 3162-96-7 with purity >99% Low price in stock
NAME:METHYL 4,6-O-BENZYLIDENE- α-D-GLUCOPYRANOSIDE
CAS No.:3162-96-7
Molecular Formula::C14H18O6
Molecular Weight:282.29
EINECS号:221-615-2
Quality Standard:
| Item |
Specification |
| Appearance |
White to off-white crystalline powder |
| Assay , % |
≥98.0 |
| Melting point, ℃ |
163.0~167.0 |
| Specific rotation [α]20D (C=1,CHCl3) |
+108.5~ +114.5° |
| Loss on drying, % |
≤1.0 |
METHYL 4,6-O-BENZYLIDENE-ALPHA-D-GLUCOPYRANOSIDE(Cas 3162-96-7) Usage
- Methyl-4, 6-benzylidene -α -D-glucopyranoside is a commonly used benzylidene protected carbohydrate derivative, usually as an intermediate, widely used in the synthesis of a variety of compounds.
The specific application is described as follows:
- By synthesizing a deacetyllanatoside glucosyl-modified compound (2-O-benzyl-beta-D-glucopyranoside-deacetyllanatoside and the like) to enhance the antitumous effect of the compound, the potency is improved, the half-life period is prolonged, meanwhile, the modified compound is prepared into a liposome, the hepatic targeting effect of the liposome is improved, and the cardiotoxicity is reduced.
- It is used for the construction of chiral trifluoromethylated quaternary carbon centres
- Synthesis of new C-6 homologues of 1-deoxynojirimycin and 1-deoxy- L –idonojirimycin.
- Use of Propargyl Glycoside for Anomeric Protection
- Synthesis of phenylurethane.
- It is used for synthesis and antimicrobial studies of pyranmycin.
- Synthesis of several Alkyl Hex-3-enopyranosiduloses.
- This product reacts with dibromomethane in aqueous solution of sodium hydroxide, using tetrabutylammonium bromide as phase transfer agent, can obtain corresponding 2, 3-methylene acetal. This product protects the 4th and 6th positions
- This product can be oxidated and ring opened by n-bromosuccinimide (NBS), and generate 6-bromo-4-O-benzoyl pyranhexose.
3162-96-7 Relevant articles
Facile Cleavage of Benzyl Ethers by Catalytic Transfer Hydrogenation
Hanessian, Stephen,Liak, Teng Jiam,Vanasse, Benoit
, p. 396 - 397 (1981)
-
Improved preparation of methyl 4,6-O-benzylidene-α-D-glucopyranoside
Spanevello, Rolando A.,Saavedra, Damian D.
, p. 460 - 461 (1999)
-
Cu-free Sonogashira Type Cross-Coupling of 6-Halo-2-cyclopropyl-3-(pyridyl-3-ylmethyl) Quinazolin-4(3H)-ones as Potential Antimicrobial Agents
Poudapally, Suresh,Gurram, Venkateshwarlu,Garlapati, Ramesh,Tulluri, Chiranjeevi,Addepally, Uma,Vidya,Sharma, Somesh,Sen, Subhabrata,Pottabathini, Narender
, p. 2272 - 2286 (2017)
C(sp)–C(sp2) bond formation via Sonogash...
Epoxy functionalized polymethacrylates based on various multifunctional D-glucopyranoside acetals
Neugebauer, Dorota,Mielanczyk, Anna,Waskiewicz, Sylwia,Biela, Tadeusz
, p. 2483 - 2494 (2013)
The synthesis of acetal-derived d-glucop...
SnCl2-Catalyzed Acetalation/Selective Benzoylation Sequence for the Synthesis of Orthogonally Protected Glycosyl Acceptors
Dong, Hai,Feng, Guang-Jing,Guo, Yang-Fan,Liu, Chun-Yang,Lv, Jian
supporting information, (2022/04/03)
Based on SnCl2-catalyzed acetalation and...
Carbohydrate-Derived Metal-Chelator-Triggered Lipids for Liposomal Drug Delivery
Holmstr?m, Thomas,Galsgaard Malle, Mette,Wu, Shunliang,Jensen, Knud J?rgen,Hatzakis, Nikos S.,Pedersen, Christian Marcus
supporting information, p. 6917 - 6922 (2021/02/26)
Liposomes are versatile three-dimensiona...
Triethylamine-methanol mediated selective removal of oxophenylacetyl ester in saccharides
Rasool, Javeed Ur,Kumar, Atul,Ali, Asif,Ahmed, Qazi Naveed
supporting information, p. 338 - 347 (2021/01/29)
A highly selective, mild, and efficient ...
Me3SI-promoted chemoselective deacetylation: a general and mild protocol
Gurawa, Aakanksha,Kashyap, Sudhir,Kumar, Manoj
, p. 19310 - 19315 (2021/06/03)
A Me3SI-mediated simple and efficient pr...
3162-96-7 Process route
-
-
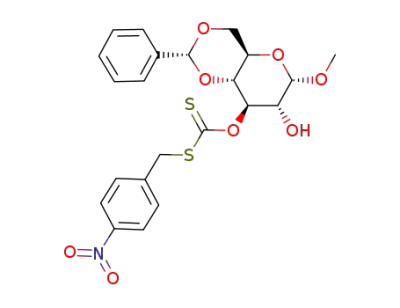
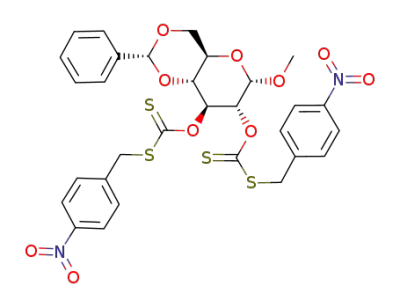
methyl 4,6-O-benzylidene-α-D-glucopyranoside-3-(S-p-nitrobenzyl-xanthate)
-
- 463-58-1
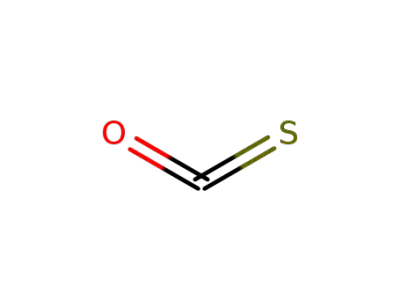
carbon oxide sulfide
-
- 26798-33-4
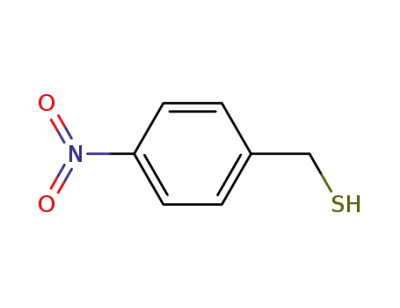
(p-nitrophenyl)methanethiol
-
- 3162-96-7
4,6-O-benzylidene-1-O-methyl-α-D-glucopyranoside
Conditions
| Conditions |
Yield |
|
With potassium chloride; water; In ethanol; at 25 ℃; Rate constant; Thermodynamic data; ΔG(excit.), ΔH(excit.), ΔS(excit.);
|
|
-
-
methyl 4,6-O-benzylidene-α-D-glucopyranoside-2,3-(S-p-nitrobenzyl-xanthate)
-
- 463-58-1
carbon oxide sulfide
-
- 26798-33-4
(p-nitrophenyl)methanethiol
-
- 3162-96-7
4,6-O-benzylidene-1-O-methyl-α-D-glucopyranoside
Conditions
| Conditions |
Yield |
|
With potassium chloride; water; In ethanol; at 25 ℃; Rate constant; Thermodynamic data; ΔG(excit.), ΔH(excit.), ΔS(excit.);
|
|
3162-96-7 Upstream products
3162-96-7 Downstream products
-
74984-87-5
Methyl-4,5-O-benzyliden-2,3-carbonato-α-D-mannopyranosid
-
120200-50-2
methyl 4,6-O-benzylidene-2,3-di-O-methyl-α-D-mannopyranoside
-
7177-79-9
methyl 2,3-di-O-benzyl-4,6-O-benzylidene-α-D-mannopyranoside
-
98392-36-0
Methyl 2-O-acetyl-4,6-O-(phenylmethylene)-α-D-mannopyranoside